The Role of Anti-Interferon-α Autoantibodies in Severe COVID-19: Implications for Vaccination Prioritization
- PMID: 40733719
- PMCID: PMC12298202
- DOI: 10.3390/vaccines13070742
The Role of Anti-Interferon-α Autoantibodies in Severe COVID-19: Implications for Vaccination Prioritization
Abstract
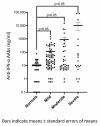
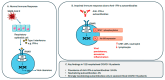
Background/Objectives: Neutralizing autoantibodies against type I interferons, particularly interferon-alpha (IFN-α), have been implicated in severe COVID-19 outcomes. This study investigated the prevalence and functional significance of anti-IFN-α autoantibodies (AAbs) in hospitalized unvaccinated COVID-19 patients and their association with COVID-19 disease severity. Methods: We retrospectively analyzed serum samples from 122 hospitalized COVID-19 patients (asymptomatic/mild: n = 69, moderate: n = 35, severe/critical: n = 18) and 32 healthy uninfected controls. Anti-IFN-α AAbs were quantified using a commercial enzyme-linked immunosorbent assay (ELISA) kit, with functional neutralization assessed via competitive ELISA and STAT1 phosphorylation inhibition. Statistical comparisons were performed using one-way ANOVA for parametric data and the Kruskal-Wallis test for non-parametric variables. Results: Anti-IFN-α AAbs were detected in 24.6% of COVID-19 patients, with all clinical subgroups showing significantly higher titers compared to healthy controls (p < 0.05). Although no significant differences in anti-IFN-α AAb levels were found between mild, moderate, and severe cases, patients with severe or critical COVID-19 had markedly higher mean titers (10,511.3 ng/mL) compared to non-severe (mild + moderate) cases (375.2 ng/mL, p < 0.001). Strongly neutralizing anti-IFN-α AAbs, with high titers (>20,000 ng/mL) and the ability to inhibit STAT1 phosphorylation, were identified in three severe COVID-19 cases. Anti-IFN-α AAb levels correlated positively with CRP (r = 0.80, p < 0.0001), LDH (r = 0.80, p = 0.001), and neutrophil count (r = 0.52, p = 0.003), and negatively with lymphocyte count (r = -0.59, p = 0.0006). Conclusions: Elevated and functionally neutralizing anti-IFN-α AAbs were associated with severe COVID-19. These findings support their role as a risk factor for poor outcomes and emphasize the importance of early COVID-19 vaccination. Screening may help identify high-risk individuals, particularly those unvaccinated or with immune vulnerabilities.
Keywords: COVID-19 vaccination; IFN-α; SARS-CoV-2; autoantibody; type I interferon.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
SARS-CoV-2-neutralising monoclonal antibodies to prevent COVID-19.Cochrane Database Syst Rev. 2022 Jun 17;6(6):CD014945. doi: 10.1002/14651858.CD014945.pub2. Cochrane Database Syst Rev. 2022. PMID: 35713300 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

