Ferritin-Based HA DNA Vaccine Outperforms Conventional Designs in Inducing Protective Immunity Against Seasonal Influenza
- PMID: 40733722
- PMCID: PMC12299999
- DOI: 10.3390/vaccines13070745
Ferritin-Based HA DNA Vaccine Outperforms Conventional Designs in Inducing Protective Immunity Against Seasonal Influenza
Abstract
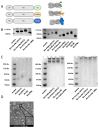
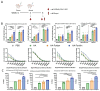
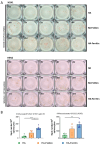
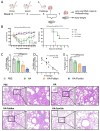
Background: Influenza remains a persistent public health challenge due to antigenic drift and shift, necessitating vaccines capable of eliciting broad and durable immunity. Hemagglutinin (HA) antigen serves as the critical target for eliciting protective immune responses against influenza. DNA vaccines offer distinct advantages over conventional platforms, including accelerated development and induction of both humoral and cellular immune responses. Methods: To optimize HA antigen presentation, we designed and systematically compared the immunogenicity and protective efficacy of HA antigen display strategies-bacteriophage T4 fibritin (HA-Foldon) and ferritin-based virus-like particles (HA-Ferritin)-versus monomeric HA DNA vaccines against seasonal influenza viruses. Results: HA-Ferritin showed superior structural stability. All vaccines induced similar HA-specific antibody levels, but HA-Ferritin elicited higher neutralizing antibodies and stronger T cell responses. Upon challenge, HA-Ferritin and HA-Foldon protected mice from weight loss and reduced lung virus loads by 3.27 and 0.76 times, respectively. Monomeric HA provided limited protection, with only 40% survival and minimal viral or pathological reduction. Conclusions: The HA-Ferritin DNA vaccine demonstrated enhanced immunogenicity and protection, supporting structured antigen display as a promising strategy for influenza DNA vaccine development.
Keywords: DNA vaccine; ferritin-based vaccine; hemagglutinin (HA) antigen; immunogenicity; influenza.
Conflict of interest statement
Author Bin Wang is the founder and chief scientific advisor of Advaccine Biopharmaceuticals Suzhou Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Iuliano A.D., Roguski K.M., Chang H.H., Muscatello D.J., Palekar R., Tempia S., Cohen C., Gran J.M., Schanzer D., Cowling B.J., et al. Estimates of Global Seasonal Influenza-Associated Respiratory Mortality: A Modelling Study. Lancet. 2018;391:1285–1300. doi: 10.1016/S0140-6736(17)33293-2. - DOI - PMC - PubMed
-
- Litvinova V.R., Rudometov A.P., Rudometova N.B., Kisakov D.N., Borgoyakova M.B., Kisakova L.A., Starostina E.V., Fando A.A., Yakovlev V.A., Tigeeva E.V., et al. DNA Vaccine Encoding a Modified Hemagglutinin Trimer of Avian Influenza A Virus H5N8 Protects Mice from Viral Challenge. Vaccines. 2024;12:538. doi: 10.3390/vaccines12050538. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

