Immune Responses Induced by Recombinant Membrane Proteins of Mycoplasma agalactiae in Goats
- PMID: 40733723
- PMCID: PMC12299757
- DOI: 10.3390/vaccines13070746
Immune Responses Induced by Recombinant Membrane Proteins of Mycoplasma agalactiae in Goats
Abstract
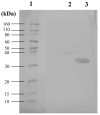
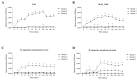
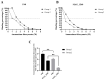
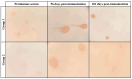
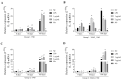
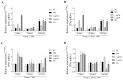
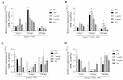
Background/Objectives: Contagious agalactia (CA) is a disease typically caused by Mycoplasma agalactiae, affecting small ruminants worldwide and being endemic in certain countries. CA causes severe economic losses due to mastitis, agalactia, and arthritis. As an alternative to existing immunoprophylactic measures, this study aimed to develop a recombinant subunit vaccine against M. agalactiae and evaluate its specific immune response in goats. Methods: Goats were divided into three groups: group 1 received recombinant proteins (P40 and MAG_1560), group 2 received formalin-inactivated M. agalactiae, and group 3 received Tris-buffered saline (negative control). All solutions were emulsified in Freund's adjuvant. Animals were monitored for 181 days. IgG antibody production was assessed by ELISA, and peripheral blood mononuclear cells (PBMCs) were analyzed by real-time PCR for the expression of IL-1β, IFN-γ, IL-12, and MHC class II genes. Results: M. agalactiae-specific antibody response was observed for six months in the sera of animals from group 1. Analysis of cytokine gene expression revealed increased IL-1β mRNA levels over time in both experimental groups. In group 1, IFN-γ mRNA levels increased with P40 stimulation and decreased with MAG_1560. IL-12 mRNA expression decreased over time in group 1 with P40 stimulation, whereas group 2 showed increased IL-12 expression for both proteins. MHC-II expression was stimulated in both groups. Conclusions: The recombinant proteins induced antibody production and cytokine expression, demonstrating immunogenic potential and supporting their promise as vaccine candidates capable of eliciting both humoral and cellular immune responses against M. agalactiae.
Keywords: M. agalactiae; contagious agalactia; recombinant; subunit; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
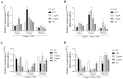
Similar articles
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Bacterial conjugation in the ruminant pathogen Mycoplasma agalactiae is influenced by eukaryotic host factors.Appl Environ Microbiol. 2025 Jun 18;91(6):e0086825. doi: 10.1128/aem.00868-25. Epub 2025 May 27. Appl Environ Microbiol. 2025. PMID: 40422289 Free PMC article.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
-
Measuring cytokines in Eurasian tundra reindeer (Rangifer tarandus tarandus) with a bovine bead-based multiplex immunoassay and real-time PCR.Acta Vet Scand. 2025 Jun 18;67(1):34. doi: 10.1186/s13028-025-00819-4. Acta Vet Scand. 2025. PMID: 40533814 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
References
-
- Gómez-Martín Á., Amores J., Paterna A., De la Fe C. Contagious Agalactia Due to Mycoplasma Spp. in Small Dairy Ruminants: Epidemiology and Prospects for Diagnosis and Control. Vet. J. 2013;198:48–56. - PubMed
-
- Madanat A., Zendulková D., Pospíšil Z. Contagious Agalactia of Sheep and Goats. A Review. Acta Vet. Brno. 2001;70:403–412. doi: 10.2754/avb200170040403. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

