Optimization of YF17D-Vectored Zika Vaccine Production by Employing Small-Molecule Viral Sensitizers to Enhance Yields
- PMID: 40733734
- PMCID: PMC12299442
- DOI: 10.3390/vaccines13070757
Optimization of YF17D-Vectored Zika Vaccine Production by Employing Small-Molecule Viral Sensitizers to Enhance Yields
Abstract
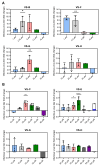
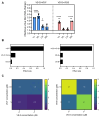
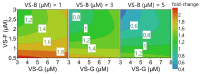
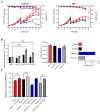
Background: Modern viral vector production needs to consider process intensification for higher yields from smaller production volumes. However, innate antiviral immunity triggered in the producer cell may limit virus replication. While commonly used cell lines (e.g., Vero or E1A-immortalised cells) are already compromised in antiviral pathways, the redundancy of innate signaling complicates host cell optimization by genetic engineering. Small molecules that are hypothesized to target antiviral pathways (Viral Sensitizers, VSEs) added to the culture media offer a versatile alternative to genetic modifications to increase permissiveness and, thus, viral yields across multiple cell lines. Methods: To explore how the yield for a chimeric Zika vaccine candidate (YF-ZIK) could be further be increased in an intensified bioprocess, we used spin tubes or an Ambr15 high-throughput microbioreactor system as scale-down models to optimize the dosing for eight VSEs in three host cell lines (AGE1.CR.pIX, BHK-21, and HEK293-F) based on their tolerability. Results: Addition of VSEs to an already optimized infection process significantly increased infectious titers by up to sevenfold for all three cell lines tested. The development of multi-component VSE formulations using a design of experiments approach allowed further synergistic titer increases in AGE1.CR.pIX cells. Scale-up to 1 L stirred-tank bioreactors and 3D-printed mimics of 200 or 2000 L reactors resulted in up to threefold and eightfold increases, respectively. Conclusions: Addition of single VSEs or combinations thereof allowed a further increase in YF-ZIK titers beyond the yield of an already optimized, highly intensified process. The described approach validates the use of VSEs and can be instructive for optimizing other virus production processes.
Keywords: antiviral defense; bioprocess engineering; design of experiments; small molecules; vectored live-attenuated vaccines; viral sensitizers.
Conflict of interest statement
Ingo Jordan and Volker Sandig are employees of ProBioGen AG, where AGE1.CR.pIX cells were developed. Kai Dallmeier is mentioned as an inventor on patent applications related to the discovery and use of YF17D-vectored vaccines. J.S., A.V., and J.D. are employed by and own shares in Virica Biotech, where the VSEs that were used were developed. M. Satzer and P. Satzer are employees of p4b GmbH, which developed the technologies used to generate the pDS down-scale systems.
Figures




References
-
- Sanchez-Felipe L., Vercruysse T., Sharma S., Ma J., Lemmens V., Van Looveren D., Javarappa M.P.A., Boudewijns R., Malengier-Devlies B., Liesenborghs L., et al. A single-dose live-attenuated YF17D-vectored SARS-CoV-2 vaccine candidate. Nature. 2021;590:320–325. doi: 10.1038/s41586-020-3035-9. - DOI - PubMed
-
- Lemmens V., Kelchtermans L., Debaveye S., Chiu W., Vercruysse T., Ma J., Thibaut H.J., Neyts J., Sanchez-Felipe L., Dallmeier K. YF17D-vectored Ebola vaccine candidate protects mice against lethal surrogate Ebola and yellow fever virus challenge. npj Vaccines. 2023;8:99. doi: 10.1038/s41541-023-00699-7. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

