Immunogenicity of DNA, mRNA and Subunit Vaccines Against Beak and Feather Disease Virus
- PMID: 40733739
- PMCID: PMC12298428
- DOI: 10.3390/vaccines13070762
Immunogenicity of DNA, mRNA and Subunit Vaccines Against Beak and Feather Disease Virus
Abstract
Background/objectives: Beak and feather disease virus (BFDV) is the causative agent of psittacine beak and feather disease (PBFD), affecting psittacine birds. There is currently no commercial vaccine or treatment for this disease. This study developed a novel BFDV coat protein mRNA vaccine encapsidated by TMV coat protein to form pseudovirions (PsVs) and tested its immunogenicity alongside BFDV coat protein (CP) subunit and DNA vaccine candidates.
Methods: mRNA and BFDV CP subunit vaccine candidates were produced in Nicotiana benthamiana and subsequently purified using PEG precipitation and gradient ultracentrifugation, respectively. The DNA vaccine candidate was produced in E. coli cells harbouring a plasmid with a BFDV1.1mer pseudogenome. Immunogenicity of the vaccine candidates was evaluated in African grey parrot chicks.
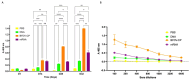
Results: Successful purification of TMV PsVs harbouring the mRNA vaccine, and of the BFDV-CP subunit vaccine, was confirmed by SDS-PAGE and western blot analysis. TEM analyses confirmed formation of TMV PsVs, while RT-PCR and RT-qPCR cDNA amplification confirmed encapsidation of the mRNA vaccine candidate within TMV particles. Restriction digests verified presence of the BFDV1.1mer genome in the plasmid. Four groups of 5 ten-week-old African grey parrot (Psittacus erithacus) chicks were vaccinated and received two boost vaccinations 2 weeks apart. Blood samples were collected from all four groups on day 14, 28 and 42, and sera were analysed using indirect ELISA, which showed that all vaccine candidates successfully elicited specific anti-BFDV-CP immune responses. The subunit vaccine candidate showed the strongest immune response, indicated by higher binding titres (>6400), followed by the mRNA and DNA vaccine candidates.
Conclusions: The candidate vaccines present an important milestone in the search for a protective vaccine against PBFD, and their inexpensive manufacture could considerably aid commercial vaccine development.
Keywords: BFDV; DNA vaccine; mRNA vaccine; subunit vaccine.
Conflict of interest statement
B.N., A.R.v.Z. and D.V. declare no conflicts of interest. E.R. and I.H. have ownership of a small shareholding in Cape Biopharms Ltd. SA (Pty) Ltd. E.R. and I.H. are patent holders of “Tobacco Mosaic Virus Pseudovirions for Stabilising Single Stranded RNA” PA176629/P. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures







Similar articles
-
Production and immunogenicity of a plant-produced beak and feather disease virus vaccine in Japanese quails.Arch Virol. 2025 Jun 25;170(7):163. doi: 10.1007/s00705-025-06352-z. Arch Virol. 2025. PMID: 40560236 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
The phylogenetic and phylogeographic landscape of the beak and feather disease virus, 1996-2022.Infect Genet Evol. 2023 Aug;112:105442. doi: 10.1016/j.meegid.2023.105442. Epub 2023 May 11. Infect Genet Evol. 2023. PMID: 37179036
-
The effect of pertussis vaccination in pregnancy on the immunogenicity of acellular or whole-cell pertussis vaccination in Gambian infants (GaPS): a single-centre, randomised, controlled, double-blind, phase 4 trial.Lancet Infect Dis. 2025 Aug;25(8):909-924. doi: 10.1016/S1473-3099(25)00072-6. Epub 2025 Mar 25. Lancet Infect Dis. 2025. PMID: 40154521 Clinical Trial.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
References
-
- Last R.D. Psittacine Beak and Feather Disease (PBFD). Refining Your Diagnostics in Aviary Birds. South African Veterinary Association; Pretoria, South Africa: 2022. pp. 1–4.
Grants and funding
- IB22-006/University of Cape Town's (UCT) Innovation Builder Fund
- 19/80/Poliomyelitis Research Foundation
- Competitive Programme for Rated Researchers/National Research Foundation
- None/UCT Doctoral Research Scholarship
- None/Council for Scientific and Industrial Research and the Department of Science and Technology (CSIR-DST)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

