Bioengineering Outer-Membrane Vesicles for Vaccine Development: Strategies, Advances, and Perspectives
- PMID: 40733744
- PMCID: PMC12298926
- DOI: 10.3390/vaccines13070767
Bioengineering Outer-Membrane Vesicles for Vaccine Development: Strategies, Advances, and Perspectives
Abstract
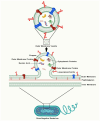
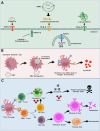
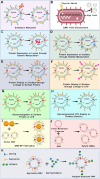
Outer-membrane vesicles (OMVs), naturally secreted by Gram-negative bacteria, have gained recognition as a versatile platform for the development of next-generation vaccines. OMVs are essential contributors to bacterial pathogenesis, horizontal gene transfer, cellular communication, the maintenance of bacterial fitness, and quorum sensing. Their intrinsic immunogenicity, adjuvant properties, and scalability establish OMVs as potent tools for combating infectious diseases and cancer. Recent advancements in genetic engineering and biotechnology have further expanded the utility of OMVs, enabling the incorporation of multiple epitopes and antigens from diverse pathogens. These developments address critical challenges such as antigenic variability and co-infections, offering broader immune coverage and cost-effective solutions. This review explores the unique structural and immunological properties of OMVs, emphasizing their capacity to elicit robust immune responses. It critically examines established and emerging engineering strategies, including the genetic engineering of surface-displayed antigens, surface conjugation, glycoengineering, nanoparticle-based OMV engineering, hybrid OMVs, and in situ OMV production, among others. Furthermore, recent advancements in preclinical research on OMV-based vaccines, including synthetic OMVs, OMV-based nanorobots, and nanodiscs, as well as emerging isolation and purification methods, are discussed. Lastly, future directions are proposed, highlighting the potential integration of synthetic biology techniques to accelerate research on OMV engineering.
Keywords: bioengineered vaccines; cancer vaccines; genetic engineering; multi-antigen vaccines; multi-pathogen vaccines; outer membrane vesicles; vaccine development.
Conflict of interest statement
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures




References
-
- Turnbull L., Toyofuku M., Hynen A.L., Kurosawa M., Pessi G., Petty N.K., Osvath S.R., Cárcamo-Oyarce G., Gloag E.S., Shimoni R. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat. Commun. 2016;7:11220. doi: 10.1038/ncomms11220. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

