Circulating Antibody's Role During Post-Exposure Prophylaxis, and Beyond for Rabies: A Review
- PMID: 40733752
- PMCID: PMC12299083
- DOI: 10.3390/vaccines13070775
Circulating Antibody's Role During Post-Exposure Prophylaxis, and Beyond for Rabies: A Review
Abstract
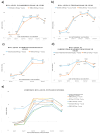
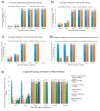
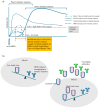
Background: Since the introduction of Pasteur's rabies vaccine in 1885, rabies prophylaxis and post-exposure prophylaxis (PEP) have been widely administered globally under the recommendation of the World Health Organization (WHO). However, 124 documented cases of PEP failure had been reported worldwide between 1980 and 2023. Additionally, sporadic media reports from China showed occasional PEP failures between 2017 and 2024. Rabies remains a serious public health problem in over 150 countries and regions. Methods: In this review, we summarize PEP procedures recommended by the Advisory Committee on Immunization Practices (ACIP) and the WHO. We also analyze potential contributing factors to PEP failure, propose a concept of circulating antibodies, and discuss their roles in PEP. Furthermore, we summarize key guidelines for clinical trial design from the U.S. Food and Drug Administration (FDA) and China's Center for Drug Evaluation (CDE), as well as the latest developments in monoclonal antibody (cocktail) therapies. Results: Adherence to core PEP practices, such as wound cleansing, infiltration of wounds with immunoglobulin (mAbs), and administration of vaccines, and broader societal involvement are crucial for preventing rabies infection in most cases. For high-risk exposures or immunocompromised individuals, the provision of circulating antibodies through high-dose human rabies immune globulin (HRIG) or mAbs is of utmost importance for preventing PEP failure. Conclusions: Early, high-concentration circulating antibodies are important for preventing PEP failure. Addressing the global issue of rabies requires involvement of the entire society. Only through collective efforts can we tackle this neglected disease and achieve WHO's goal of "zero by 30".
Keywords: PEP failure; circulating antibody; monoclonal antibodies; rabies.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- WHO Rabies. [(accessed on 1 June 2025)]. Available online: https://www.who.int/news-room/fact-sheets/detail/rabies.
-
- Rupprecht C.E., Briggs D., Brown C.M., Franka R., Katz S.L., Kerr H.D., Lett S., Levis R., Meltzer M.I., Schaffner W., et al. Evidence for a 4-dose vaccine schedule for human rabies post-exposure prophylaxis in previously non-vaccinated individuals. Vaccine. 2009;27:7141–7148. doi: 10.1016/j.vaccine.2009.09.029. - DOI - PubMed
-
- Manning S.E., Rupprecht C.E., Fishbein D., Hanlon C.A., Lumlertdacha B., Guerra M., Meltzer M.I., Dhankhar P., Vaidya S.A., Jenkins S.R., et al. Human rabies prevention—United States, 2008: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm. Rep. 2008;57:1–28. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

