Unidirectional Transmembrane Photoinduced Electron Transfer with Artificial Metallopeptides
- PMID: 40734722
- PMCID: PMC12302060
- DOI: 10.1021/aps.5c00010
Unidirectional Transmembrane Photoinduced Electron Transfer with Artificial Metallopeptides
Abstract
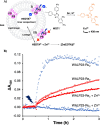
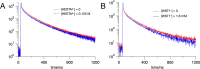
The ability to drive unidirectional photoinduced electron transfer through an insulating lipid membrane is one of the most striking features of the thylakoid membrane, and recreating this property artificially is considered as one of the holy grails of artificial photosynthesis. Here, we report two artificial metallopeptides, WALP23-Re 2 and WALP23-Ru 2 , that were embedded in the membrane of dissymmetric liposomes containing an electron donor in the inner compartment and an electron acceptor in the bulk aqueous phase. Upon light irradiation under air, unidirectional electron transfer was observed from one side of the membrane to the other one with both metallopeptides. However, the mechanism of photoinduced electron transfer strongly depended on the metal center: the neutral WALP23-Re 2 peptide achieved genuine electron transfer through the membrane, but with the tetracationic WALP23-Ru 2 peptide the transmembrane electron transfer appeared to be the result of light-induced leakage of the electron donor molecules through the lipid bilayer, followed by electron transfer on the same side of the membrane by peptides assembled parallel to the membrane. These results highlight both the unique potential of the neutral metallopeptide WALP23-Re2 to drive transmembrane photoinduced electron transfer in artificial photosynthetic systems, as well as the importance of membrane-leakage studies to validate the working mechanism of such supramolecular systems.
Keywords: artificial photosynthesis; leakage; lipid bilayers; photoelectron transfer; transmembrane.
© 2025 The Authors. Co-published by Dalian Institute of Chemical Physics, CAS, Westlake University, and American Chemical Society.
Figures



Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Robinson J. N., Cole-Hamilton D. J.. Electron Transfer across Vesicle Bilayers. Chem. Soc. Rev. 1991;20(1):49–94. doi: 10.1039/cs9912000049. - DOI
LinkOut - more resources
Full Text Sources
