Countrywide introduction of pulsed field ablation for the treatment of atrial fibrillation: Acute results from the FRANCE-PFA registry
- PMID: 40734736
- PMCID: PMC12302170
- DOI: 10.1016/j.hroo.2025.04.005
Countrywide introduction of pulsed field ablation for the treatment of atrial fibrillation: Acute results from the FRANCE-PFA registry
Abstract
Background: Most data on atrial fibrillation (AF) ablation using the first available pentaspline pulsed field ablation (PFA) catheter (Farapulse, Boston Scientific Inc) come from retrospective center-level registries collected in highly experienced centers.
Objective: This study aimed to provide exhaustive and prospective patient-level data on this new ablation modality.
Methods: FRANCE-PFA is a nationwide registry (NCT06497933) that included all patients undergoing a first AF ablation using the pentaspline PFA catheter since the introduction of this technology in France. All French centers using this technology participated. Procedural data were prospectively collected at a patient level.
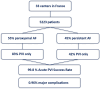
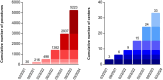
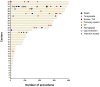
Results: This registry included 5223 patients from 33 centers between March 2021 and February 2024 (mean age 65 ± 11 years, 55.4% paroxysmal AF). The procedure duration was 54 ± 23 minutes. Acute pulmonary vein isolation was achieved in 5211 patients (99.8%). The total number of PFA applications was 50 ± 22 with >70 applications in 746 patients (14.3%). Pulmonary vein isolation only was performed in 64.7% of patients (82.7% of paroxysmal AF, 44.5% of persistent AF, and 26.6% of long-standing persistent AF). The most common location for additional PFA lesion sets was the left atrium posterior wall in 1335 patients (25.6%), left atrium roof in 999 patients (19.1%), and mitral isthmus in 514 patients (9.8%). Major complications occurred in 50 patients (0.96%), with no esophageal complication or symptomatic phrenic nerve palsy reported in past hospital discharge.
Conclusion: In this prospective and nationwide registry, AF ablation using the pentaspline PFA catheter seemed to be safe and acutely efficient, despite considerable heterogeneity in the number of patients treated at each center.
Trial registration: NCT06497933.
Keywords: Atrial fibrillation; Catheter ablation; Pulmonary vein isolation; Pulsed field ablation; Registry.
© 2025 Heart Rhythm Society. Published by Elsevier Inc.
Figures


References
-
- Joglar J.A., Chung M.K., Armbruster A.L., et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2024;149:e1–e156. - PMC - PubMed
-
- Van Gelder I.C., Rienstra M., Bunting K.V., et al. ESC guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): developed by the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC), with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Endorsed by the European Stroke Organization (ESO) Eur Heart J. 2024;2024:ehae176.
-
- Reddy V.Y., Koruth J., Jais P., et al. Ablation of atrial fibrillation with pulsed electric fields: an ultra-rapid, tissue-selective modality for cardiac ablation. JACC Clin Electrophysiol. 2018;4:987–995. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

