Synergistic seizure reduction in patient with persistently elevated N-desmethylclobazam levels, CYP450 genetic polymorphism, and responsive neurostimulator targeting centromedian nuclei of bilateral thalami
- PMID: 40734774
- PMCID: PMC12304684
- DOI: 10.1016/j.ebr.2025.100799
Synergistic seizure reduction in patient with persistently elevated N-desmethylclobazam levels, CYP450 genetic polymorphism, and responsive neurostimulator targeting centromedian nuclei of bilateral thalami
Abstract
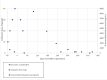
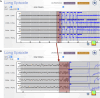
Clobazam (CLB) and cenobamate (CNB) are commonly used antiseizure medications (ASMs) in the treatment of patients with drug-resistant epilepsy (DRE). However, concomitant use of these two ASMs may lead to significant treatment-related adverse events (TRAE). Furthermore, these TRAE may be exacerbated in individuals with genetic polymorphisms involving the P450 system. In patients with DRE, epilepsy surgery, including neuromodulation, may lead to improved seizure control and a reduction in systemic TRAE from ASMs. This case report describes a patient with drug-resistant idiopathic generalized epilepsy (IGE) who experienced persistent excessive somnolence correlated with elevated N-desmethylclobazam (N-CLB) levels. Pharmacogenetic testing revealed poor metabolism of CYP2C19, and N-CLB levels remained elevated and detectable for nearly one year after the discontinuation of treatment with CLB and CNB. Responsive neurostimulator (RNS) implantation within the bilateral centromedian nuclei (CMN) of the thalamus resulted in seizure freedom until N-CLB levels fell, after which there was an 83-93 % reduction in the frequency of generalized tonic-clonic seizures (GTC).
Keywords: Cenobamate; Clobazam; Idiopathic generalized epilepsy; Pharmacogenetics; Responsive neurostimulator.
© 2025 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Andrew Zillgitt has received payments as part of speaker bureaus for UCB (brivaracetam and midazolam), Eisai and Catalyst (perampanel), SK Lifesciences (cenobamate), and Jazz Pharmaceuticals (cannabidiol). He has received payments for consulting for UCB and Catalyst. Dr. David Burdette has received payments as part of speaker bureaus for UCB (brivaracetam, midazolam, and fenfluramine), Eisai and Catalyst (perampanel), SK Lifesciences (cenobamate), Jazz Pharmaceuticals (cannabidiol), and NeuroPace. He has contracted research with NeuroPace and Longbaord. Dr. Michael Staudt has received payments for consulting for Boston Scientific, Medtronic, NeuroOne, and Nevro. Drs. Atheel Yako and Sydney Jacobs as well as Revati Rashingkar, MS3, and Ashleigh Terrell, NP-C have no financial or other disclosures.
Figures

References
-
- Chapman A.G., Horton R.W., Meldrum B.S. Anticonvulsant action of a 1,5-benzodiazepine, clobazam, in reflex epilepsy. Epilepsia. 1978;19(3):293–299. - PubMed
-
- Gastaut H., Low M.D. Antiepileptic properties of clobazam, a 1,5-benzodiazepine, in man. Epilepsia. 1979;20:437–446. - PubMed
-
- Nakamura F., Suzuki S., Nishimura S., Yagi K., Seino M. Effects of clobazam and its active metabolite on GABA-activated currents in rat cerebral neurons in culture. Epilepsia. 1996;37(8):728–735. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
