Impact of Precision-Guided Dosing on Clinical Decision-Making and Health Care Utilization in Inflammatory Bowel Disease: A Retrospective Pretest/Posttest Real-World Study
- PMID: 40735050
- PMCID: PMC12305531
- DOI: 10.1093/crocol/otaf044
Impact of Precision-Guided Dosing on Clinical Decision-Making and Health Care Utilization in Inflammatory Bowel Disease: A Retrospective Pretest/Posttest Real-World Study
Abstract
Background: Precision-guided dosing (PGD) is a personalized tool that optimizes clinical decision-making in the treatment of inflammatory bowel disease (IBD) with infliximab (IFX) and its biosimilars. PGD employs nonlinear mixed-effect models using patient-specific pharmacokinetic parameters to predict infliximab trough concentrations without the need to wait until the actual trough measurement. This approach calculates patient-specific clearance (CL) and provides tailored IFX dosing and administration intervals aimed at achieving target trough levels. Implementing PGD can enhance treatment outcomes in IBD patients and may potentially reduce healthcare expenditures.
Methods: We conducted a multicenter, retrospective study as a follow up to our previous clinical experience program (CEP). We aimed to evaluate the impact of PGD on clinical decision-making, patient outcomes, healthcare utilization, and expenditures. Treatment decisions included: IFX dose intensification, reduction, discontinuation, or continuation. Disease activity and healthcare resource utilization and costs in the 12 months pre- and post-test were compared. Disease activity was measured using the physician global assessment (PGA) as follows: remission (0), mild (1), moderate (2), and severe (3). Costs were calculated based on modeling pre-established literature data.
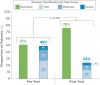
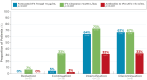
Results: Analysis of data from 82 patients across 7 states and Puerto Rico showed that PGD-driven therapeutic decision making led to IFX treatment intensification (27%) or discontinuation (7%) in patients with low forecasted trough IFX concentrations, high clearance, and presence of antidrug antibody. Conversely, IFX dosage was reduced (18%) or unchanged (48%) for patients with high IFX concentrations and low clearance. There was a significant association between forecasted trough IFX levels and treatment modifications (P < .001). High clearance (> 0.294 L/day) was significantly associated with therapy intensification (OR 6.22, 95% CI: 2.19-19.8; P < .001). Following PGD, disease activity improved significantly (observed mean difference in physician global assessment: 0.378, P = 0.008) and healthcare resource utilization decreased. Across the entire patient population, hospitalizations decreased from 30 events pretest to 5 events posttest (P < .001), leading to overall cost saving.
Conclusions: HCPs used the PGD test to guide treatment decisions. PGD-driven optimization of IFX therapy led to improved patient outcomes, lower healthcare utilization, and cost savings.
Keywords: dose optimization; healthcare costs; novel laboratory developed test; pharmacokinetics; precision medicine; precision-guided dosing; therapeutic drug monitoring.
© The Author(s) 2025. Published by Oxford University Press on behalf of Crohn's & Colitis Foundation.
Conflict of interest statement
T.D. and P.A.H. are employees of Prometheus Laboratories Inc. All other authors received fair market value compensation from Prometheus Laboratories Inc. for their participation in retrospective chart reviews that supported data collection for this research.
Figures

References
-
- Kotla NG, Rochev Y.. IBD disease-modifying therapies: insights from emerging therapeutics. Trends Mol Med. 2023;29(3):241-253. doi: https://doi.org/ 10.1016/j.molmed.2023.01.001 - DOI - PubMed
-
- Baumgart DC, Sandborn WJ.. Crohn’s disease. Lancet. 2012;380(9853):1590-1605. doi: https://doi.org/ 10.1016/S0140-6736(12)60026-9 - DOI - PubMed
-
- Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ.. Ulcerative colitis. Lancet. 2012;380(9853):1606-1619. doi: https://doi.org/ 10.1016/S0140-6736(12)60150-0 - DOI - PubMed
-
- Turner D, Ricciuto A, Lewis A, et al. ; International Organization for the Study of IBD. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology. 2021;160(5):1570-1583. doi: https://doi.org/ 10.1053/j.gastro.2020.12.031 - DOI - PubMed
-
- Colombel JF, D’Haens G, Lee WJ, Petersson J, Panaccione R.. Outcomes and strategies to support a treat-to-target approach in inflammatory bowel disease: a systematic review. J Crohns Colitis. 2020;14(2):254-266. doi: https://doi.org/ 10.1093/ecco-jcc/jjz131 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
