Insights from the trial innovation network's initial consultation process
- PMID: 40735123
- PMCID: PMC12305380
- DOI: 10.1017/cts.2025.10084
Insights from the trial innovation network's initial consultation process
Abstract
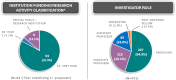
Multicenter clinical trials are essential for evaluating interventions but often face significant challenges in study design, site coordination, participant recruitment, and regulatory compliance. To address these issues, the National Institutes of Health's National Center for Advancing Translational Sciences established the Trial Innovation Network (TIN). The TIN offers a scientific consultation process, providing access to clinical trial and disease experts who provide input and recommendations throughout the trial's duration, at no cost to investigators. This approach aims to improve trial design, accelerate implementation, foster interdisciplinary teamwork, and spur innovations that enhance multicenter trial quality and efficiency. The TIN leverages resources of the Clinical and Translational Science Awards (CTSA) program, complementing local capabilities at the investigator's institution. The Initial Consultation process focuses on the study's scientific premise, design, site development, recruitment and retention strategies, funding feasibility, and other support areas. As of 6/1/2024, the TIN has provided 431 Initial Consultations to increase efficiency and accelerate trial implementation by delivering customized support and tailored recommendations. Across a range of clinical trials, the TIN has developed standardized, streamlined, and adaptable processes. We describe these processes, provide operational metrics, and include a set of lessons learned for consideration by other trial support and innovation networks.
Keywords: Multicenter studies; Recruitment Innovation Center; Trial Innovation Center; Trial Innovation Network; scientific consultation.
© The Author(s) 2025.
Conflict of interest statement
Dr Benjamin reported personal fees from AbbVie, PPD, and Syneos Health outside the submitted work. No other disclosures were reported.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
