Membrane Contact Sites in Proteostasis and ER Stress Response
- PMID: 40735229
- PMCID: PMC12304649
- DOI: 10.1177/25152564251363050
Membrane Contact Sites in Proteostasis and ER Stress Response
Abstract
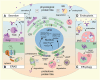
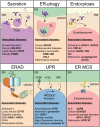
Execution of all cellular functions depends on a healthy proteome, whose maintenance requires multimodal oversight. Roughly a third of human proteins reside in membranes and thus present unique topological challenges with respect to biogenesis and degradation. To meet these challenges, eukaryotes have evolved organellar pathways of protein folding and quality control. Most transmembrane proteins originate in the endoplasmic reticulum (ER), where they are subject to surveillance and, if necessary, removal through either ER-associated proteasomal degradation (cytosolic pathway) or selective autophagy (ER-phagy; organellar pathway). In the latter case, ER cargoes are shuttled to (endo)lysosomes - the same organelles that degrade cell surface molecules via endocytosis. Here, we provide an overview of dynamic coordination between the ER and endolysosomes, with a focus on their engagement in specialized physical interfaces termed membrane contact sites (MCSs). We cover how cross-compartmental integration through MCSs allows biosynthetic and proteolytic organelles to fine-tune each other's membrane composition, organization, and dynamics and facilitates recovery from proteotoxic stress. Along the way, we highlight recent developments and open questions at the crossroads between organelle biology and protein quality control and cast them against the backdrop of factor-specific diseases associated with perturbed membrane homeostasis.
Keywords: endolysosome; endoplasmic reticulum; membrane contact sites; proteostasis; proteotoxic stress.
© The Author(s) 2025.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
-
- Alpy F, Rousseau A, Schwab Y, Legueux F, Stoll I, Wendling C, Spiegelhalter C, Kessler P, Mathelin C, Rio MC, Levine TP. (2013). STARD3 Or STARD3NL and VAP form a novel molecular tether between late endosomes and the ER. J Cell Sci 126(Pt 23), 5500–5512. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
