Disulfidptosis-Related Genes as Novel Biomarkers and Therapeutic Targets in Dilated Cardiomyopathy
- PMID: 40735269
- PMCID: PMC12306564
- DOI: 10.2147/JIR.S525114
Disulfidptosis-Related Genes as Novel Biomarkers and Therapeutic Targets in Dilated Cardiomyopathy
Abstract
Background: Dilated cardiomyopathy (DCM) is a severe cardiac condition characterized by ventricular dilation and impaired systolic function, leading to heart failure and sudden death. Current treatments have limited efficacy in reversing disease progression. Recent research suggests that disulfidptosis, a novel cell death mechanism, may play a role in DCM pathogenesis, though its specific involvement remains unclear.
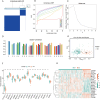
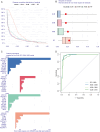
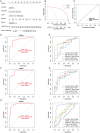
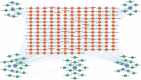
Methods: This study utilized multiple GEO datasets to analyze the expression of 24 disulfidptosis-related genes (DiGs) in DCM patients and healthy controls. Differential expression analysis and consensus clustering were used to classify DCM patients into subgroups based on DiGs expression profiles. A predictive model was constructed using four machine learning methods, and its performance was validated with independent datasets. A ceRNA regulatory network was established, and in vivo experiments were conducted using a DCM mouse model to validate key findings.
Results: Nineteen DiGs were differentially expressed between DCM and controls. Consensus clustering divided DCM patients into two subtypes with distinct immune profiles. The support vector machine (SVM) model demonstrated superior diagnostic performance (AUC = 0.983), identifying ACTN4, MYH10, TLN1, DSTN, and NCKAP1 as core predictors. In vivo validation confirmed their expression changes in myocardial tissue. Single-cell RNA-seq further revealed increased fibroblast and macrophage infiltration in DCM, along with enrichment of fibrotic and immune pathways. Quercetin and resveratrol were predicted as potential drugs targeting these core genes.
Conclusion: This study demonstrates that ACTN4, MYH10, TLN1, DSTN, and NCKAP1 play significant roles in the pathogenesis of DCM, influencing immune cell infiltration and metabolic homeostasis. Quercetin and resveratrol were identified as potential therapeutic agents, offering new directions for DCM treatment.
Keywords: diagnostic model; dilated cardiomyopathy; disulfidptosis-related genes; immune cell infiltration.
© 2025 Bo et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Disulfidptosis-related gene in acute myocardial infarction and its diagnostic value and functions based on bioinformatics analysis and machine learning.Front Cardiovasc Med. 2025 Jul 2;12:1513342. doi: 10.3389/fcvm.2025.1513342. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40672380 Free PMC article.
-
Identification of immune-related biomarkers linked to systemic lupus erythematosus and dilated cardiomyopathy through integrated bioinformatics analysis and multiple machine learning algorithms.Front Immunol. 2025 Jul 30;16:1606920. doi: 10.3389/fimmu.2025.1606920. eCollection 2025. Front Immunol. 2025. PMID: 40808946 Free PMC article.
-
Frequency of autoantibodies and their associated clinical characteristics and outcomes in patients with dilated cardiomyopathy: A systematic review and meta-analysis.Autoimmun Rev. 2025 Mar 26;24(4):103755. doi: 10.1016/j.autrev.2025.103755. Epub 2025 Jan 22. Autoimmun Rev. 2025. PMID: 39855285
-
Construction of a Disulfidptosis-Related Prediction Model for Acute Myocardial Infarction Based on Transcriptome Data.Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2025 Jun 30;47(3):354-365. doi: 10.3881/j.issn.1000-503X.16057. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2025. PMID: 40704466
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
References
-
- Stephane H, Neal KL, Carsten T, Karin K. Dilated cardiomyopathy: causes, mechanisms, and current and future treatment approaches. Lancet. 2023;402(10406):998–1011. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

