GLP-1 receptor agonists in IBD: exploring the crossroads of metabolism and inflammation
- PMID: 40735319
- PMCID: PMC12306662
- DOI: 10.3389/fimmu.2025.1610368
GLP-1 receptor agonists in IBD: exploring the crossroads of metabolism and inflammation
Abstract
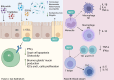
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) represent a cornerstone in the treatment of diabetes and obesity and have emerged as a promising option for other metabolic disorders, including hepatic steatosis. Recent evidence highlights the direct and indirect anti-inflammatory properties of GLP-1, suggesting a potential additional therapeutic strategy for patients with inflammatory bowel disease (IBD). However, side effects of GLP-1 RAs, particularly those affecting the gastrointestinal system, may limit their use in patients with IBD. The rising prevalence of IBD worldwide and the ageing of the IBD population will likely increase the number of patients with metabolic comorbidities who may potentially benefit from a combination treatment with GLP-1 RAs. A profound comprehension of the physiological function of intestinal homeostasis and permeability is essential to more accurately evaluate the prospective application of GLP-1 RAs in patients with ongoing inflammation. While preclinical studies support this hypothesis, robust clinical evidence remains limited. This narrative review aims to provide a synthesis of current knowledge regarding the anti-inflammatory properties of GLP-1, with a particular focus on safety concerns and potential future directions for its use in IBD management.
Keywords: GLP-1; GLP-1 receptor agonists; IBD; diabetes; obesity.
Copyright © 2025 Migliorisi, Gabbiadini, Dal Buono, Ferraris, Privitera, Petronio, Bertoli, Bezzio and Armuzzi.
Conflict of interest statement
Author AA has received consulting fees from AbbVie, Allergan, Amgen, Arena, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion, Eli-Lilly, Ferring, Galapagos, Gilead, Janssen, MSD, Mylan, Pfizer, Protagonist Therapeutics, Roche, Samsung Bioepis, Sandoz and Takeda; speaker’s fees from AbbVie, Amgen, Arena, Biogen, Bristol-Myers Squibb, Eli-Lilly, Ferring, Galapagos, Gilead, Janssen, MSD, Novartis, Pfizer, Roche, Samsung Bioepis, Sandoz, Takeda, and Tigenix; and research support from Biogen, MSD, Takeda, and Pfizer. Author CB received lecture fees and served as a consultant for Takeda, MSD, Ferring, Abbvie, Galapagos and Janssen. R. Author RB has received speaker’s fees from Pfizer, MSD, Celltrion and Takeda. Author AB has received speaker’s fees from AbbVie, Galapagos, Celltrion and Pfizer. G. Author GP has received speaker’s fees from Janssen and Alphasigma. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Singh S, George J, Boland BS, Vande Casteele N, Sandborn WJ. Primary non-response to tumor necrosis factor antagonists is associated with inferior response to second-line biologics in patients with inflammatory bowel diseases: A systematic review and meta-analysis. J Crohns Colitis. (2018) 12:635–43. doi: 10.1093/ecco-jcc/jjy004 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

