The effect of tranexamic acid on blood coagulation in primary total hip arthroplasty using rotational thromboelastometry: a randomized controlled trial
- PMID: 40735345
- PMCID: PMC12304718
- DOI: 10.1016/j.eclinm.2025.103374
The effect of tranexamic acid on blood coagulation in primary total hip arthroplasty using rotational thromboelastometry: a randomized controlled trial
Abstract
Background: Tranexamic acid (TXA), is commonly administered prophylactically to reduce blood loss in patients undergoing total hip arthroplasty (THA). However, its effect has never been studied. We hypothesized that no difference exists in the degree of fibrinolysis and blood loss between patients receiving prophylactic TXA and placebo.
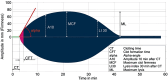
Methods: This double-blinded randomized-controlled trial included 50 patients undergoing primary THA in 2021-2023. Clinicaltrials.gov (NCT03897621). Rotational-thromboelastometry (ROTEM) were performed to test blood coagulability using non citrated whole blood (NATEM) and blood treated with TXA (T-APTEM). The intervention group received TXA intravenously. The placebo group received 0.9% sodium chloride solution. The primary outcome measure was to quantitate the degree of fibrinolysis measured by maximum lysis (ML) demonstrated by ROTEM variables. Fibrinolysis was defined as ML (maximum lysis) > 15% within 1 h of testing.
Findings: Blood coagulability tested by ROTEM was within the normal range in all patients, and no difference was found between the TXA group and placebo group.NATEM and T-APTEM variables were similar in both groups and no patient developed fibrinolysis during the entire perioperative phases. At baseline, T-APTEM, compared with NATEM, showed shorter CT (746 ± 265 vs. 991 ± 237 p < 0.05) and greater ML (1.9 ± 2.2 vs. 0.8 ± 1, p < 0.05), suggesting some degree of acceleration of coagulation. Postoperatively, blood coagulability showed a tendency of acceleration with shorter CT (689 ± 188 vs. 828 ± 163, p < 0.05) and CFT (258 ± 101 vs. 293 ± 87 p < 0.05) and increased A10 (41 ± 9 vs. 38 ± 8, p < 0.05). Clinical outcomes, including blood loss, hematologic variables, and coagulation profile were similar between the two groups.
Interpretation: Normal range of blood coagulability in all patients, no significant differences between NATEM and T-APTEM variables, and similar clinical outcome between the two groups suggest that there is no definitive medical indication for TXA administration in patients undergoing THA without a preexisting fibrinolytic condition. Monitoring blood coagulability using ROTEM may be useful in guiding selective administration of TXA in high-risk patients.
Funding: Department of Anesthesiology funding, Thomas Jefferson University Hospital. Support was provided solely from institutional and/or departmental sources.
Keywords: Antifibrinolytic; Coagulation; Fibrinolysis; Rotational thromboelastometry; Total hip arthroplasty; Tranexamic acid.
© 2025 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
UY: Leadership–International Liver Transplantation Society. MT: Grants–RTM Vital Signs LLC Grant, NIH SBIR Grant. Pending Patents–Non invasive respiratory monitor With Jefferson and RTM Vital Signs LLC. JM: Support for attending meetings–Thomas Jefferson University. Leadership–ASRA Perioperative Special Interest Group – Co-Chair (Unpaid).
Figures
References
-
- Fillingham Y.A., Ramkumar D.B., Jevsevar D.S., et al. The efficacy of tranexamic acid in total hip arthroplasty: a network meta-analysis. J Arthroplasty. 2018;33:3083–3089. - PubMed
-
- Sentilhes L., Sénat M.V., Le Lous M., et al. Tranexamic acid for the prevention of blood loss after cesarean delivery. N Engl J Med. 2021;384:1623–1634. - PubMed
-
- Devereaux P.J., Marcucci M., Painter T.W., et al. POISE-3 Investigators Tranexamic acid in patients undergoing noncardiac surgery. N Engl J Med. 2022;386:1986–1997. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical