Low-dose trimethoprim-sulfamethoxazole for prophylaxis of Pneumocystis jirovecii pneumonia in HIV-uninfected patients: a systematic review and meta-analysis
- PMID: 40735480
- PMCID: PMC12303816
- DOI: 10.3389/fphar.2025.1545436
Low-dose trimethoprim-sulfamethoxazole for prophylaxis of Pneumocystis jirovecii pneumonia in HIV-uninfected patients: a systematic review and meta-analysis
Abstract
Background: Trimethoprim-sulfamethoxazole (TMP-SMX) is the recommended first-line prophylactic agent against Pneumocystis jirovecii pneumonia (PJP). However, the standard regimen is often discontinued due to its drug-associated adverse events (AEs), especially in immunocompromised patients without HIV infection. Therefore, we aimed to investigate the efficacy and safety of a low-dose regimen of TMP-SMX against PJP prophylaxis in patients without infection.
Methods: We searched PubMed, Embase, Wanfang, China National Knowledge Infrastructure, Web of Science, and the Cochrane database for relevant articles from inception to 15 October 2024. Studies were included if they reported the safety and efficacy of using TMP-SMX in PJP prophylaxis in patients without HIV infection. The primary outcome was the discontinuation rate. We assessed study quality and performed sensitivity and subgroup analysis to explore potential heterogeneity among the included studies.
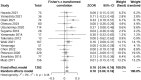
Results: Seventeen studies with 4,890 patients were included. These studies were low to modest in quality. Overall, the incidence of PJP in the included studies was rare and was similar between the low- and standard-dose groups. However, the low-dose regimen significantly reduced the risk of discontinuation rate (odds ratio [OR] = 0.38; 95% CI, 0.27-0.52; I 2 = 0%; P < 0.00001). Further sensitivity and subgroup analyses confirmed this finding. Estimation of the combined discontinuation rate for patients receiving low-dose TMP-SMX was 10% (95% CI, 4%-16%). The low-dose regimen also significantly reduced total AEs (OR = 0.33; 95% CI, 0.24-0.46; I 2 = 22%; P < 0.00001) and improved the incidence of most specific AEs (ORs ranged from 0.24 to 0.67), especially in outcomes of fever, rash, thrombocytopenia, hyponatremia, and liver and renal function (P values ranged from 0.0001 to 0.02).
Conclusion: Our findings suggested that a low-dose TMP-SMX regimen is safe and significantly reduces the discontinuation rate and total AEs compared to the standard regimen against PJP in HIV-uninfected patients. Thus, it is a potentially promising prophylactic regimen, and more well-designed, high-quality research should be conducted.
Systematic review registration: https://inplasy.com/inplasy-2024-4-0084/.
Keywords: Pneumocystis jirovecii pneumonia; discontinuation rate; meta-analysis; prophylaxis; trimethoprim-sulfamethoxazole.
Copyright © 2025 Huang, Shi, Hu, Zhu and Yu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Antinori A., Murri R., Ammassari A., De Luca A., Linzalone A., Cingolani A., et al. (1995). Aerosolized pentamidine, cotrimoxazole and dapsone-pyrimethamine for primary prophylaxis of Pneumocystis carinii pneumonia and toxoplasmic encephalitis. AIDS Lond. Engl. 9 (12), 1343–1350. 10.1097/00002030-199512000-00007 - DOI - PubMed
-
- Bozzette S. A., Finkelstein D. M., Spector S. A., Frame P., Powderly W. G., He W., et al. (1995). A randomized trial of three antipneumocystis agents in patients with advanced human immunodeficiency virus infection. NIAID AIDS clinical trials group. N. Engl. J. Med. 332 (11), 693–699. 10.1056/NEJM199503163321101 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

