Living Kidney Donation Practices in Europe: A Survey of DESCaRTES and EKITA Transplantation Working Groups
- PMID: 40735507
- PMCID: PMC12303855
- DOI: 10.3389/ti.2025.14802
Living Kidney Donation Practices in Europe: A Survey of DESCaRTES and EKITA Transplantation Working Groups
Abstract
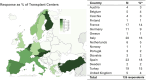
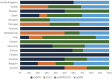
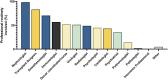
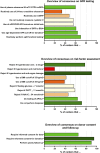
Thorough evaluation of potential kidney donors ensures safety and graft quality, but European data on donor practices are lacking. An online survey was conducted to assess European practices regarding kidney function, risk assessment and follow-up. 56% of respondents (125 practitioners, 16 countries, ∼3700 donations annually) use eGFRCKD-EPI, 34% use creatinine clearance and 70% use measured GFR. Sixty-three percent have no upper age limits, 91% exclude candidates with hypertension with end-organ damage, and 78% candidates on ≥2 antihypertensives. BMI cut-offs of 30 (39%) and 35 kg/m2 (42%) are common. Candidates are excluded for an HbA1c ≥ 53 mmol/mol (46%), glucose ≥7 (57%) or ≥11.1 mmol/L after glucose-tolerance test (59%). ApoL1-testing is not routine in 73%, and 38% perform a kidney biopsy if albuminuria/hematuria is present. Spot and 24-hour urine albumin is assessed in 38%. Hematuria is accepted when urological evaluation (15%), kidney biopsy (16%), or both (57%) are normal. Low-risk stones often do not preclude donation. Written informed consent is obtained by 95% of centers, with 65% asking consent for data. Lifetime follow-up is offered by 83%. This first study on evaluation and follow-up practices of donors in Europe shows variation between centers, suggesting a need for harmonization of donor practices.
Keywords: donor follow-up; donor screening; kidney function; living kidney donation; risk assessment.
Copyright © 2025 van Londen, Gaillard, Zaza, Oniscu, Gandolfini, Furian, Stojanovic, Cucchiari, Hilbrands, Mjøen and Mariat.
Conflict of interest statement
This study was conducted on behalf of the DESCaRTES working group of the European Renal Association and the EKITA working group of the European Society for Organ Transplantation.
Figures

References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

