Construction of EGCG/chlorhexidine functionalized coating to reinforce the soft tissue seal at transmucosal region of implants
- PMID: 40735522
- PMCID: PMC12304417
- DOI: 10.1093/rb/rbaf046
Construction of EGCG/chlorhexidine functionalized coating to reinforce the soft tissue seal at transmucosal region of implants
Abstract
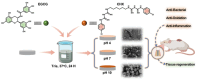
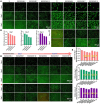
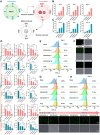
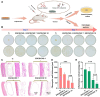
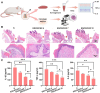
Recent advancements in dental implant technology have provided more reliable and durable solutions for patients. Soft tissue seal (STS) is crucial for achieving implant stability, maintaining tissue health and promoting integration with surrounding soft and hard tissues. However, the STS around implants is fragile and susceptible to disruption by oral pathogens, particularly in patients with periodontitis or poor oral hygiene, leading to complications such as peri-implant mucositis and peri-implantitis. To promote STS formation, it is crucial to maintain the balance between bacterial and host cells while effectively managing inflammation. Although titanium-based implants exhibit biocompatibility, they lack inherent antibacterial and anti-inflammatory properties. To address these challenges, we developed a dual-function antibacterial and anti-inflammatory coating using chlorhexidine (CHX) and epigallocatechin gallate (EGCG). CHX effectively reduces bacterial adhesion but may inhibit fibroblast proliferation, while EGCG provides antioxidant and anti-inflammatory benefits. Three types of EGCG/CHX composite coatings were developed on titanium surfaces at different pH values. These coatings exhibited enhanced bacterial resistance, reduced inflammation and ROS scavenging capabilities, with higher pH levels further improving their performance. In vivo studies also confirmed that these coatings effectively prevented bacterial adhesion, mitigated inflammation and promoted STS formation, thereby holding significant promise for enhancing the long-term success of dental implants.
Keywords: STS; anti-inflammatory; antibacterial; dental implant.
© The Author(s) 2025. Published by Oxford University Press.
Figures




References
-
- Jiang X, Yao Y, Tang W, Han D, Zhang L, Zhao K, Wang S, Meng Y. Design of dental implants at materials level: an overview. J Biomed Mater Res A 2020;108:1634–61. - PubMed
-
- Zhou N, Dong H, Zhu Y, Liu H, Zhou N, Mou Y. Analysis of implant loss risk factors especially in maxillary molar location: a retrospective study of 6977 implants in Chinese individuals. Clin Implant Dent Relat Res 2019;21:138–44. - PubMed
LinkOut - more resources
Full Text Sources

