The Visualization of Nocturnal Scratching Behavior in Pediatric Atopic Dermatitis: Facilitating Shared Decision-Making and Assessing the Efficacy of Treatment
- PMID: 40735660
- PMCID: PMC12306594
- DOI: 10.7759/cureus.88988
The Visualization of Nocturnal Scratching Behavior in Pediatric Atopic Dermatitis: Facilitating Shared Decision-Making and Assessing the Efficacy of Treatment
Abstract
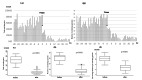
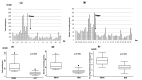
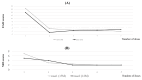
Itching is a subjective symptom that is especially difficult to assess in pediatric atopic dermatitis (AD) patients with chronic pruritus since early infancy. This study aimed to assess the utility of the Itch Tracker (Maruho Co. Ltd., Osaka, Japan), a smartwatch application, in visualizing nocturnal scratching and facilitating shared decision-making (SDM) in two pediatric AD cases treated with nemolizumab. Objective monitoring revealed discrepancies between self-reported itch and actual scratching behavior, leading to appropriate treatment initiation and significant improvements in scratching behavior, Eczema Area and Severity Index (EASI), and numerical rating scale (NRS) scores. These results highlight the clinical value of behavioral visualization in enhancing SDM and optimizing management in pediatric AD.
Keywords: atopic dermatitis; itch tracker; nemolizumab; scratching; shared decision-making.
Copyright © 2025, Sugiyama et al.
Conflict of interest statement
Human subjects: Informed consent for treatment and open access publication was obtained or waived by all participants in this study. The Ethics Committee of the National Hospital Organization Fukuoka National Hospital issued approval F5-7. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: Takeshi Nakahara declare(s) personal fees from Takeshi Nakahara has received consulting fees, honoraria, grant support, or lecturing fees from AbbVie, LEO Pharma, Maruho, Mitsubishi Tanabe Pharma, Otsuka Pharma, Sanofi, Sun Pharma, Taiho Pharmaceutical, and Torii Pharmaceutical Co., Ltd . Akiko Sugiyama declare(s) personal fees from Maruho Co., Ltd. Akiko Sugiyama has received honoraria from Maruho Co., Ltd and received the Takagi Clinical Research Development Award from Maruho Takagi Dermatology Foundation. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- The neurobiology of itch. Ikoma A, Steinhoff M, Ständer S, Yosipovitch G, Schmelz M. Nat Rev Neurosci. 2006;7:535–547. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
