Topography-based implants for bone regeneration: Design, biological mechanism, and therapeutics
- PMID: 40735704
- PMCID: PMC12305250
- DOI: 10.1016/j.mtbio.2025.102066
Topography-based implants for bone regeneration: Design, biological mechanism, and therapeutics
Abstract
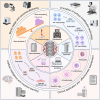
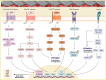
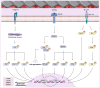
With the increasing demand for bone defect repair, bone implant materials have emerged as a critical alternative to traditional autologous or allogeneic bone grafts. However, their clinical performance remains limited due to challenges such as prolonged healing times and suboptimal repair quality. Moreover, in patients with certain pathological conditions (e.g., diabetes mellitus and osteoporosis), disruptions in the bone microenvironment further compromise regenerative outcomes. To address these limitations, surface modification strategies have been developed to regulate implant-bone tissue interactions and improve therapeutic efficacy. This review systematically summarizes recent advances in bone regeneration implants with a focus on topographical modifications, encompassing design principles, underlying biological mechanisms, and therapeutic applications. Particular attention is given to the influence of implant surface topography on the biological behaviors of osteoblasts, osteoclasts, and macrophages within the bone microenvironment, as well as their responses under complex pathological and physiological conditions. The review also discusses current challenges related to achieving micro/nanoscale structural balance, personalization, and clinical translation of implant surface topographies, and highlights future directions in precision bone regeneration through multidisciplinary approaches, artificial intelligence-driven optimization, and long-term clinical validation. Collectively, these insights may inform future research on bone implant materials and support the development of novel strategies for personalized treatment of bone defect repair.
Keywords: Biological mechanisms; Bone regeneration; Implants; Surface morphology; Translational applications.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








Similar articles
-
A Narrative review of advancements in knee Arthroplasty: Analyzing diverse prosthetic materials and their implications.J Clin Orthop Trauma. 2025 Apr 24;66:103034. doi: 10.1016/j.jcot.2025.103034. eCollection 2025 Jul. J Clin Orthop Trauma. 2025. PMID: 40433656 Review.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Property-tailoring chemical modifications of hyaluronic acid for regenerative medicine applications.Acta Biomater. 2025 Jul 1;201:75-100. doi: 10.1016/j.actbio.2025.06.014. Epub 2025 Jun 7. Acta Biomater. 2025. PMID: 40490241 Review.
-
Interventions to improve safe and effective medicines use by consumers: an overview of systematic reviews.Cochrane Database Syst Rev. 2014 Apr 29;2014(4):CD007768. doi: 10.1002/14651858.CD007768.pub3. Cochrane Database Syst Rev. 2014. PMID: 24777444 Free PMC article.
References
-
- Li W., Su Z., Hu Y., Meng L., Zhu F., Xie B., Zhou Z., Cui S., Wang M., Wu Q., Yao S. Functional and structural construction of photothermal-responsive PEEK composite implants to promote bone regeneration and bone-implant integration. Compos. Sci. Technol. 2024;258 doi: 10.1016/j.compscitech.2024.110885. - DOI
Publication types
LinkOut - more resources
Full Text Sources

