Disease outcomes following lateral switch among different CD20-antibodies in active multiple sclerosis
- PMID: 40735835
- PMCID: PMC12357975
- DOI: 10.1177/13524585251361330
Disease outcomes following lateral switch among different CD20-antibodies in active multiple sclerosis
Abstract
Background: Ocrelizumab (OCR) and ofatumumab (OFA)are approved and their differences in dosing route and interval allow personalized treatment. However, there are no data on whether lateral switches between both substances affect treatment effectiveness or safety.
Methods: We screened our local cohort of MS patients, who began OCR since 09/2020 or OFA since 09/2021. Patients with a lateral switch were matched to controls who continuously received initial B-cell depleting therapy (BCT). We compared disease courses including effectiveness outcomes as well as peripheral CD19+ B-cell counts and serum IgG levels.
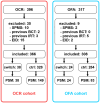
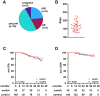
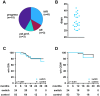
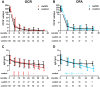
Results: From 09/2020 to 03/2024, 713 patients were subjected to BCT (OCR: 396; OFA: 317 [as in Fig.1]). The matched OCR cohort included 38 switchers and 149 controls. The OFA cohort consisted of 24 switchers and 83 controls. Effectiveness outcomes were comparable among switchers and controls. B cell depletion appeared slightly pronounced following a switch. Serum IgG levels declined faster among switchers compared to controls (OCR: 9.7 vs 9.0 g/L; p = 0.007; manifest hypogammaglobulinemia (HGG) in 13.2% vs 6.0%; OFA: 9.7 vs 8.4 g/L; p = 0.016; manifest HGG in 8.3% vs 2.4%).
Conclusions: Lateral switching between BCT does not abate effectiveness in this matched real-world cohort. Our observation of increased loss of IgG warrants further validation, but may indicate niche-specific immunological effects of OFA and OCR.
Keywords: Multiple sclerosis; hypogammaglobulinemia; lateral switch; ocrelizumab; ofatumumab.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Franziska Axhausen: declares no conflicts of interest.Anne Mrochen: received honoraria for lecturing and consulting from Alexion and Roche and travel reimbursements from Alexion.Pia Winter: declares no conflicts of interest.Romy Baumgart: received travel reimbursements from Sanofi-Aventis.Pauline Mühlenbrock: declares no conflicts of interest.Anna Mück: declares no conflicts of interest.Hagen B. Huttner: declares no conflicts of interest.Stephanie Wolff: received honoraria for lecturing from Mylan and Novartis and received research support from Novartis.Sven G. Meuth: received honoraria for lecturing, travel expenses and for attending meetings from Academy 2, Argenx, Alexion, Almirall, Amicus Therapeutics Germany, AstraZeneca, Bayer Health Care, Biogen, BioNtech, BMS, Celgene, Datamed, Demecan, Desitin, Diamed, Diaplan, DPmed, Gen Medicine and Healthcare products, Genzyme, Hexal AG, IGES, Impulze GmbH, Janssen Cilag, KW Medipoint, MedDay Pharmaceuticals, Medmile, Merck Serono, MICE, Mylan, Neuraxpharm, Neuropoint, Novartis, Novo Nordisk, ONO Pharma, Oxford PharmaGenesis, QuintilesIMS, Roche, Sanofi, STADA, Chugai Pharma, Teva, UCB, Viatris, Wings for Life international and Xcenda. He received research support from Alexion, Almirall, Amicus Therapeutics Germany, Argenx, Bayer Vital GmbH, BGP Products Operations (Viatris Company), Biogen, BMS, Demecan, Diamed, Fresenius Medical Care, Genzyme, HERZ Burgdorf, Hexal, Janssen, Merck Serono, Novartis, Novo Nordisk Pharma, ONO Pharma, Roche and Teva.Kathrin Möllenhoff: declares no conflicts of interest.Steffen Pfeuffer: received honoraria for lecturing and consulting from argenx, Sanofi-Avents, Merck Healthcare, Biogen, Novartis Pharma, Roche, Alexion and Hexal, travel reimbursements from Sanofi-Aventis, Merck Healthcare, Novartis Pharma, Roche, and Biogen, and research funding from Merck Healthcare, Novartis Pharma and Biogen.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

