Cathelicidin regulates goblet cell mucus secretion and mucus-associated proteins in Citrobacter rodentium-induced colitis
- PMID: 40735968
- PMCID: PMC12320862
- DOI: 10.1080/19490976.2025.2538696
Cathelicidin regulates goblet cell mucus secretion and mucus-associated proteins in Citrobacter rodentium-induced colitis
Abstract
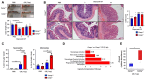
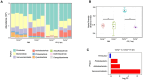
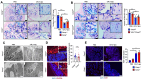
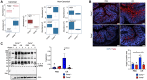
Colonic goblet cells play a crucial role in mucosal defense by secreting Muc2 mucin and other proteins that entrap and expel enteropathogens. However, the role of innate effectors in the gut like cathelicidin peptides in regulating the mucus barrier during infections remains unclear. In this study, we used cathelicidin-deficient (Camp-/-) littermates, colonoids, and human LS174T goblet-like cells to investigate how cathelicidin modulates goblet cell function and mucosal defense against attaching/effacing enteropathogen Citrobacter rodentium. We showed that increased fecal shedding and epithelial colonization by C. rodentium in Camp-/- littermates was accompanied by impaired mucus secretion and higher retention of mucin granules and trefoil factor 3 (Tff3) in bloated colonic goblet cells. Reduction in mucus secretion by goblet cells was accompanied by reduced reactive oxygen species (ROS) production during C. rodentium infection in Camp-/- as compared to Camp+/+ littermate controls. In LS174T goblet-like cells, human cathelicidin LL-37 stimulated the secretion of TFF3 and resistin-like molecule β (RELMβ) in a ROS-dependent manner. These findings reveal that cathelicidin regulates goblet cell mucus and mucus-associated protein secretion through a ROS-mediated mechanism critical for bacterial clearance and maintenance of gut homeostasis.
Keywords: Colonic epithelium; cathelicidin; citrobacter rodentium; goblet cells; mucus; reactive oxygen species.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures





References
-
- Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB, et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLOS Pathogens. 2010;6(5):e1000902. doi: 10.1371/journal.ppat.1000902. - DOI - PMC - PubMed
-
- Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Büller HA, Dekker J, Van Seuningen I, Renes IB, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131(1):117–129. doi: 10.1053/j.gastro.2006.04.020. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
