Transcutaneous spinal random noise stimulation enhances motor memory consolidation and corticospinal transmission in humans
- PMID: 40736043
- PMCID: PMC12369297
- DOI: 10.1113/JP287804
Transcutaneous spinal random noise stimulation enhances motor memory consolidation and corticospinal transmission in humans
Abstract
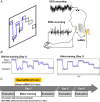
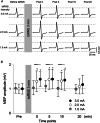
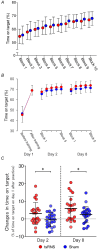
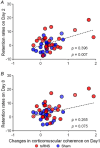
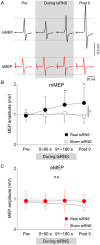
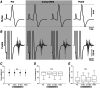
Stochastic resonance sensory input modulates the central nervous system's excitability, thereby possibly influencing motor skill learning and retention. We investigated the effects of transcutaneous spinal random noise stimulation (tsRNS) at the cervical level on motor skill learning and corticospinal transmission in healthy humans. Participants performed a 20 min visuomotor tracking training task requiring rapid shifts in pinch force, with motor performance tests conducted before, immediately after, 1 day after and 7 days after the training to assess motor skill learning and retention. During the task, participants received real or sham tsRNS for 20 and 0.5 min, respectively. Motor performance improved equally in both groups immediately after training; however, the real tsRNS group showed a higher performance than the sham group at 1 and 7 days post-training. Beta-band corticomuscular coherence increased immediately after training in both groups, and higher performance on 1 day after the training was positively correlated with a greater change in corticomuscular coherence. To elucidate the mechanisms contributing to the enhanced motor memory retention induced by tsRNS, we investigated its effects on cortical and spinal excitability. We observed increased intracortical facilitation and somatosensory evoked potential amplitude following tsRNS; however, the efficacy of cortico-motoneuronal synaptic transmissions and the excitability of spinal motoneurons remained unchanged. Collectively, tsRNS can enhance the corticospinal drive to spinal motoneurons indirectly by increasing the ascending afferent input strength and cortical excitability via the augmented activity of facilitatory interneurons, resulting in improved motor memory retention. Thus, tsRNS may have important clinical applications for rehabilitation after central nervous system lesions. KEY POINTS: Stochastic resonance sensory input modulates the excitability of the central nervous system and may influence motor skill learning and motor memory retention. Transcutaneous spinal random noise stimulation (tsRNS) applied at the cervical level can enhance motor skill learning and motor memory retention in healthy humans. tsRNS can increase the ascending afferent input to the cortex and the excitability of the intracortical circuits rather than directly modulating the descending motor output, resulting in improved motor memory retention. These findings suggest that tsRNS is an effective strategy for promoting functional motor recovery of the upper limb after the development of central nervous system lesions.
Keywords: motor skill learning; precision grip; rehabilitation; spinal stimulation; transcranial magnetic stimulation; upper limb.
© 2025 The Author(s). The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Abe, T. , Miyaguchi, S. , Otsuru, N. , & Onishi, H. (2019). The effect of transcranial random noise stimulation on corticospinal excitability and motor performance. Neuroscience Letters, 705, 138–142. - PubMed
-
- Allison, T. , McCarthy, G. , Wood, C. C. , Darcey, T. M. , Spencer, D. D. , & Williamson, P. D. (1989). Human cortical potentials evoked by stimulation of the median nerve. I. Cytoarchitectonic areas generating short‐latency activity. Journal of Neurophysiology, 62(3), 694–710. - PubMed
-
- Allison, T. , McCarthy, G. , Wood, C. C. , & Jones, S. J. (1991). Potentials evoked in human and monkey cerebral cortex by stimulation of the median nerve. A review of scalp and intracranial recordings. Brain, 114(6), 2465–2503. - PubMed
-
- Butler, J. E. , & Thomas, C. K. (2003). Effects of sustained stimulation on the excitability of motoneurons innervating paralyzed and control muscles. Journal of Applied Physiology, 94(2), 567–575. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
