The chromosome-level genome assembly of the slug Deroceras laeve facilitates its use as a comparative model of regeneration
- PMID: 40736079
- PMCID: PMC12506671
- DOI: 10.1093/g3journal/jkaf164
The chromosome-level genome assembly of the slug Deroceras laeve facilitates its use as a comparative model of regeneration
Abstract
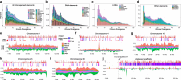
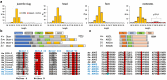
The genome of the land slug Deroceras laeve was sequenced, assembled up to the chromosome level, and annotated for non-coding RNAs and protein-coding genes. Due to the small size of this pulmonate species, ease of laboratory culture, cosmopolitan distribution, as well as recently released anatomical and histological resources, this genomic resource creates new opportunities for the investigation of the largely unexplored mechanisms of regeneration in mollusks. Moreover, it also makes this slug an attractive model for functional genomics and evolutionary biology.
Keywords: Genome Assembly; Hi-C; PacBio; Stylommatophora; cognate chromosomes; mollusk.
© The Author(s) 2025. Published by Oxford University Press on behalf of The Genetics Society of America.
Conflict of interest statement
Conflicts of interest: The authors state that no conflicts of interest exist.
Figures






References
-
- Accorsi A et al. 2024. A new genetically tractable non-vertebrate system to study complete camera-type eye regeneration. Pages: 2024.01.26.577494 Section: New Results.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
