In Vivo Wound Healing and Immune Response Studies of Chitosan Cryogels With Invertebrate Model Organism Galleria mellonella
- PMID: 40736166
- PMCID: PMC12309346
- DOI: 10.1002/bip.70042
In Vivo Wound Healing and Immune Response Studies of Chitosan Cryogels With Invertebrate Model Organism Galleria mellonella
Abstract
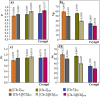
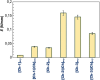
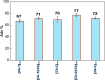
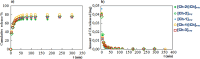
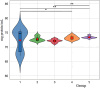
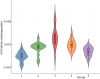
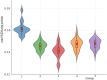
In the present study, it was aimed to prepare single and double network chitosan (Ch) cryogels cross-linked with glutaraldehyde (G), which can be recommended for use as model wound dressings and hemostatic agents, and to reveal in vivo studies with Galleria mellonella. An in vivo study about Ch cryogels with these larvae was not declared in the literature, so our study is the first of its kind. G. mellonella was used to determine the effects of cryogels on immunity, oxidative stress, and wound healing. Cinnamic acid (CA) was loaded onto the cryogels, and the percent cumulative release data of CA were found to be in the range of 69%-80%. The results show that loading of CA onto [Ch-3]cry cryogels considerably improved immune responses; the [Ch-3]cry-CA group was the most successful in terms of immunological response, oxidative stress balance, and wound healing. In accordance with the 3R principles of ethical animal research, the use of G. mellonella in this study served as a scientifically relevant and ethically responsible alternative model to mammals for preliminary assessment of wound healing potential and innate immune activation. The porous structures, high mechanical strengths, and rapidly swelling-deswelling abilities of [Ch-2@Ch]cry and [Ch-3]cry cryogels indicated that these may be suitable for biomedical applications. Analysis of SEM micrographs indicated that the morphology of dual network cryogels prepared in the form of interpenetrating polymeric networks (IPNs) was more regular and homodispersed with respect to single network cryogels. The compressive elasticity modulus (E) values of IPNs cryogels (0.160 N/mm) is approximately 4.6 times that of Ch cryogels with a single network (0.035 N/mm).
Keywords: G. mellonella; chitosan cryogel; full‐IPN cryogel; hemostatic material; wound dressings.
© 2025 The Author(s). Biopolymers published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures











References
-
- Wen J., Weinhart M., Lai B., Kizhakkedathu J., and Brooks D. E., “Reversible Hemostatic Properties of Sulfabetaine/Quaternary Ammonium Modified Hyperbranched Polyglycerol,” Biomaterials 86 (2016): 42–55. - PubMed
-
- Rezvani Ghomi E., Khalili S., Nouri Khorasani S., Esmaeely Neisiany R., and Ramakrishna S., “Wound Dressings: Current Advances and Future Directions,” Journal of Applied Polymer Science 136 (2019): 47738.
-
- Howe N. and Cherpelis B., “Obtaining Rapid and Effective Hemostasis,” Journal of the American Academy of Dermatology 69 (2013): 659.e1–659.e17. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

