PCAIs stimulate MAPK, PI3K/AKT pathways and ROS-Mediated apoptosis in aromatase inhibitor-resistant breast cancer cells while disrupting actin filaments and focal adhesion
- PMID: 40736275
- PMCID: PMC12309409
- DOI: 10.18632/oncotarget.28759
PCAIs stimulate MAPK, PI3K/AKT pathways and ROS-Mediated apoptosis in aromatase inhibitor-resistant breast cancer cells while disrupting actin filaments and focal adhesion
Abstract
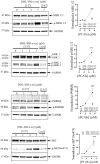
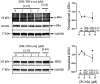
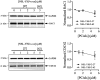
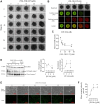
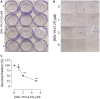
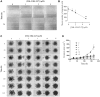
The estrogen receptor is overexpressed in and promotes 67-80% and 90% of female and male breast cancer cases, respectively. Hormone independence, enhanced motility, and signaling by growth factors have been attributed to aromatase inhibitor (AI) resistance and MAPK pathway activation. We used long-term letrozole-treated (LTLT-Ca) breast cancer cells to evaluate polyisoprenylated cysteinyl amide inhibitors (PCAIs) as potential therapies for AI-resistant breast cancer. PCAIs specifically disrupt G-proteins such as KRAS, an upstream regulator of MAPK and PI3K/AKT pathways. PCAIs were tested against the viability, phosphorylation of MAPK and PI3K/AKT pathways, apoptosis, and migration of LTLT-Ca cells. NSL-YHJ-2-27 was potent against LTLT-Ca viability with an EC50 of 4.8 μM. MEK (p-MEK1/2), ERK (p-ERK1/2), and p90RSK (p-p90RSK) phosphorylation were significantly increased by 2-, 2-, and 6.4-fold, respectively. PCAIs increased AKT phosphorylation 36-fold. NSL-YHJ-2-27 at 2, 3 and 5 μM stimulated ROS generation by 4-, 8- and 10-fold, respectively. PCAIs inhibited cell proliferation and colony formation by 95% and 74%, respectively, increased active caspase 7 and BAX 1.5-fold and 56%, respectively. NSL-YHJ-2-27 (10 μM) induced LTLT-Ca spheroid degeneration by 61%. LTLT-Ca cell migration was inhibited by 31 and 80% following treatment with 2 and 5 μM NSL-YHJ-2-27, respectively. NSL-YHJ-2-27 disrupted F-actin filaments, vinculin punctates and levels by 33%. These results indicate that the PCAIs' activation of the MAPK and PI3K/AKT pathways causes apoptosis, possibly through proapoptotic p-p90RSK isoforms, AKT-induced ROS production or anoikis through disruption of focal adhesion. These effects against LTLT-Ca cells suggest potential PCAIs therapeutic applications against antihormonal-resistant breast cancers.
Keywords: LTLT-Ca cells; MAPK; PCAIs; PI3K/AKT; ROS.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection of data, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
Treatment of a mutant KRAS lung cancer cell line with polyisoprenylated cysteinyl amide inhibitors activates the MAPK pathway, inhibits cell migration and induces apoptosis.PLoS One. 2024 Oct 22;19(10):e0312563. doi: 10.1371/journal.pone.0312563. eCollection 2024. PLoS One. 2024. PMID: 39436906 Free PMC article.
-
Activation of MAP Kinase Pathway by Polyisoprenylated Cysteinyl Amide Inhibitors Causes Apoptosis and Disrupts Breast Cancer Cell Invasion.Biomedicines. 2024 Feb 20;12(3):470. doi: 10.3390/biomedicines12030470. Biomedicines. 2024. PMID: 38540084 Free PMC article.
-
Hesperetin Inhibits Bladder Cancer Cell Proliferation and Promotes Apoptosis and Cycle Arrest by PI3K/AKT/FoxO3a and ER Stressmitochondria Pathways.Curr Med Chem. 2025;32(19):3879-3904. doi: 10.2174/0109298673283888231217174702. Curr Med Chem. 2025. PMID: 38357946
-
Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD003370. doi: 10.1002/14651858.CD003370.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2009 Oct 07;(4):CD003370. doi: 10.1002/14651858.CD003370.pub3. PMID: 17253488 Updated.
-
Hormonal therapies for early breast cancer: systematic review and economic evaluation.Health Technol Assess. 2007 Jul;11(26):iii-iv, ix-xi, 1-134. doi: 10.3310/hta11260. Health Technol Assess. 2007. PMID: 17610808
References
-
- Drummond AE, Swain CTV, Brown KA, Dixon-Suen SC, Boing L, van Roekel EH, Moore MM, Gaunt TR, Milne RL, English DR, Martin RM, Lewis SJ, Lynch BM. Linking Physical Activity to Breast Cancer via Sex Steroid Hormones, Part 2: The Effect of Sex Steroid Hormones on Breast Cancer Risk. Cancer Epidemiol Biomarkers Prev. 2022; 31:28–37. 10.1158/1055-9965.EPI-21-0438. - DOI - PMC - PubMed
-
- Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, and Panel members. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22:1736–47. 10.1093/annonc/mdr304. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

