Early and opposing neutrophil and CD4 T cell responses shape pulmonary tuberculosis pathology
- PMID: 40736456
- PMCID: PMC12309470
- DOI: 10.1084/jem.20250161
Early and opposing neutrophil and CD4 T cell responses shape pulmonary tuberculosis pathology
Abstract
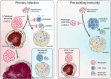
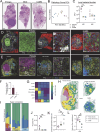
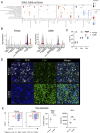
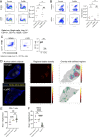
Pulmonary Mycobacterium tuberculosis (Mtb) infection results in a variety of heterogeneous lesion structures, from necrotic granulomas to alveolitis, but the mechanisms regulating their development remain unclear. Using a mouse model of concomitant immunity and subsequent aerosol infection, we demonstrate that counter regulation between neutrophils and CD4 T cells occurs very early during infection and governs these distinct pathologies. In primary Mtb infection, a dysregulated feed-forward circuit of neutrophil recruitment occurs, in which neutrophils hinder CD4 T cell interactions with infected macrophages, cause granuloma necrosis, and establish a replicative niche that drives a two-log increase in lung bacterial burden. Conversely, the rapid recruitment and activation of T cells due to concomitant immunity promotes local macrophage activation and dampens detrimental neutrophil responses. Together, these studies uncover fundamental determinants of tuberculosis lung pathology, which have important implications for new strategies to prevent or treat tuberculosis.
© 2025 Gern et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures







Update of
-
CD4-mediated immunity shapes neutrophil-driven tuberculous pathology.bioRxiv [Preprint]. 2024 Apr 15:2024.04.12.589315. doi: 10.1101/2024.04.12.589315. bioRxiv. 2024. Update in: J Exp Med. 2025 Oct 6;222(10):e20250161. doi: 10.1084/jem.20250161. PMID: 38659794 Free PMC article. Updated. Preprint.
References
-
- Barrios-Payán, J., Aguilar-León D., Lascurain-Ledezma R., and Hernández-Pando R.. 2006. [Neutrophil participation in early control and immune activation during experimental pulmonary tuberculosis]. Gac Med. Mex. 142:273–281. - PubMed
-
- Benator D., Bhattacharya M., Bozeman L., Burman W., Cantazaro A., Chaisson R., Gordin F., Horsburgh C.R., Horton J., Khan A., et al. 2002. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: A randomised clinical trial. Lancet. 360:528–534. 10.1016/S0140-6736(02)09742-8 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

