Efficacy and Safety of Low-Dose (0.2 mg) Dutasteride for Male Androgenic Alopecia: A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Phase III Clinical Trial
- PMID: 40736519
- PMCID: PMC12318778
- DOI: 10.5021/ad.25.048
Efficacy and Safety of Low-Dose (0.2 mg) Dutasteride for Male Androgenic Alopecia: A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Phase III Clinical Trial
Abstract
Background: Dutasteride, a 5-alpha reductase inhibitor, is prescribed for male androgenetic alopecia (AGA) in Korea and Japan. Despite its efficacy, its use is limited by its long half-life, potent dihydrotestosterone suppression, and adverse effects.
Objective: To investigate the efficacy and safety of 0.2 mg dutasteride for male AGA.
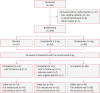
Methods: Patients with male AGA were randomized to receive 0.2 mg dutasteride, placebo, or 0.5 mg dutasteride (2:2:1) once daily for 24 weeks. Safety and efficacy endpoints were assessed.
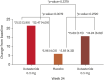
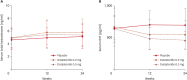
Results: Overall, 139 men were analyzed. At week 24, the change in hair count within the target area at the vertex from baseline was significantly higher in the 0.2 mg dutasteride group than in the placebo group (21.53 vs. 5.96, p=0.0072). Dutasteride (0.2 mg) treatment led to greater hair growth improvement, as assessed by investigators at week 24 (p=0.0096) and an independent panel at weeks 12 and 24 (p=0.0306, p=0.0001). For all efficacy endpoints, 0.2 mg dutasteride was as effective as 0.5 mg dutasteride. The incidence of adverse events was low and not statistically different between the 0.2 mg dutasteride and placebo groups. The limitation of this study is the limited number of participants.
Conclusion: Low-dose (0.2 mg) dutasteride for male AGA showed significant efficacy and favorable safety profile.
Trial registration: ClinicalTrials.gov Identifier: NCT04825561.
Keywords: 5-Alpha reductase inhibitor; Androgenic alopecia; Dutasteride.
© 2025 The Korean Dermatological Association and The Korean Society for Investigative Dermatology.
Conflict of interest statement
The trial was designed by the sponsor, Addpharma Co., Ltd., which collected and analyzed the data.
Figures

References
-
- Rathnayake D, Sinclair R. Male androgenetic alopecia. Expert Opin Pharmacother. 2010;11:1295–1304. - PubMed
-
- Hamilton JB. Patterned loss of hair in man; types and incidence. Ann N Y Acad Sci. 1951;53:708–728. - PubMed
-
- Paik JH, Yoon JB, Sim WY, Kim BS, Kim NI. The prevalence and types of androgenetic alopecia in Korean men and women. Br J Dermatol. 2001;145:95–99. - PubMed
-
- Chen W, Zouboulis CC, Orfanos CE. The 5α-reductase system and its inhibitors. Recent development and its perspective in treating androgen-dependent skin disorders. Dermatology. 1996;193:177–184. - PubMed
-
- Inui S, Itami S. Molecular basis of androgenetic alopecia: from androgen to paracrine mediators through dermal papilla. J Dermatol Sci. 2011;61:1–6. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

