Proteome of oocyte spindle identifies Ccdc69 regulates spindle assembly like "band-tightening spell"
- PMID: 40736675
- PMCID: PMC12311101
- DOI: 10.1007/s00018-025-05821-7
Proteome of oocyte spindle identifies Ccdc69 regulates spindle assembly like "band-tightening spell"
Abstract
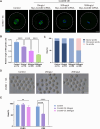
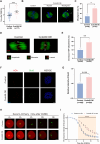
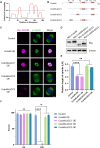
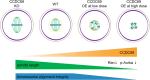
Meiotic spindle is an intricate structure and required for chromosome segregation and the proper meiotic progression during oocyte maturation, and its function is regulated by a complex network of proteins located at spindle and its peripheral region. However, proteome of meiotic spindle remains poorly characterized. Here, we acquired the proteomic profile of spindles isolated from metaphase I (MI) and metaphase II (MII) mouse oocytes. In particular, we identified Ccdc69 as a novel regulator of spindle assembly in mouse oocytes. Although deletion of Ccdc69 did not affect female fertility, the MI spindles were elongated in Ccdc69 knockout oocytes. Overexpression of Ccdc69 induced spindle defects by reducing microtubule formation and disturbing acentriolar microtubule organization centers (aMTOCs) distribution. Furthermore, Ccdc69 overexpression impaired kinetochore-microtubule (K-MT) attachment and delayed meiotic progression by abnormal activation of spindle assembly checkpoint (SAC). Taken together, our study depicts the proteome of spindles during mouse oocyte maturation and demonstrates that Ccdc69 regulates spindle assembly and meiotic progression the way similar to "The Tightening Spell of Sun Wukong's Golden Headband" in the famous Chinese Classic Journey to the West.
Keywords: Ccdc69; Meiosis; Oocyte; Proteome; Spindle assembly.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Mice were feed in specific pathogen-free barrier facilities at experimental animal center of the Institute of Zoology (IOZ), Chinese Academy of Science (Beijing, China). All animal handling protocols were approved by the guidelines of Animal Research Committee of the Institute of Zoology. Consent for publication: Not applicable. Conflict of interest: The authors declare that they have no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

