Immobilized β-galactosidase BgaC from Bifidobacterium adolescentis retains stability and activity during repeated cycles of use
- PMID: 40736680
- PMCID: PMC12310786
- DOI: 10.1007/s00253-025-13564-5
Immobilized β-galactosidase BgaC from Bifidobacterium adolescentis retains stability and activity during repeated cycles of use
Abstract
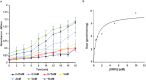
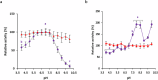
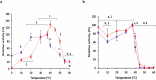
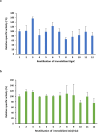
β-Galactosidase enzymes catalyze the hydrolysis of terminal non-reducing β-D-galactose residues in β-galactosides. These enzymes are important in producing lactose-free dairy products, reducing the lactose content of whey in dairy products, and for production of galactooligosaccharides (GOS) as prebiotic additives to infant formula. To use β-galactosidases in industrial settings, enzyme immobilization procedures are used to enhance their activity and stability and to minimize enzyme quantities and cost. In this study, recombinant Bifidobacterium adolescentis β-galactosidase BgaC was immobilized in calcium alginate and gelatin cross-linked with glutaraldehyde. The kinetic parameters and stability properties of immobilized BgaC were characterized in comparison with free soluble enzyme. The KM for immobilized BgaC using ortho-nitrophenyl-β-galactoside (ONPG) was 810 ± 220 μM and the KM of free BgaC was 2500 ± 3 μM. The kcat and kcat/KM of immobilized BgaC were 802 s-1 and 990 s-1 mM-1, respectively, compared to kcat and kcat/KM values of 209 s-1 and 84 s-1 mM-1, respectively, for free BgaC. Immobilized BgaC β-galactosidase was active at all tested pH (pH 4-10), while the free enzyme had decreased activity at pH < 5.5 and > 8.0. The immobilized enzyme had optimum activity at 40 °C, while the free enzyme was most active at 37 °C. In addition, immobilization enhanced acidic pH and temperature stability compared to the free enzyme. Reutilization of the BgaC beads was assessed and the enzyme maintained 69% activity after 12 rounds of reutilization. Therefore, the enhanced performance properties of immobilized BgaC make it a promising candidate for industrial applications. KEY POINTS: • Bifidobacterium adolescentis β-galactosidase BgaC was successfully immobilized • Immobilized BgaC has enhanced enzymatic activity and stability and allows recycling • Sustained activity of immobilized BgaC is advantageous for industrial applications.
Keywords: BgaC; Bifidobacterium; Calcium alginate; Entrapment; Immobilization; β-Galactosidase.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Metagenomic identification, purification and characterisation of the Bifidobacterium adolescentis BgaC β-galactosidase.Appl Microbiol Biotechnol. 2021 Feb;105(3):1063-1078. doi: 10.1007/s00253-020-11084-y. Epub 2021 Jan 11. Appl Microbiol Biotechnol. 2021. PMID: 33427933 Free PMC article.
-
Streamlined production of immobilized D-psicose 3-epimerase via secretion in Pichia pastoris: a new paradigm for industrial D-psicose production.Microb Cell Fact. 2025 Jul 2;24(1):149. doi: 10.1186/s12934-025-02763-4. Microb Cell Fact. 2025. PMID: 40598404 Free PMC article.
-
CALB Immobilized on Octyl-Agarose-An Efficient Pharmaceutical Biocatalyst for Transesterification in Organic Medium.Int J Mol Sci. 2025 Jul 20;26(14):6961. doi: 10.3390/ijms26146961. Int J Mol Sci. 2025. PMID: 40725207 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Classic Galactosemia and Clinical Variant Galactosemia.2000 Feb 4 [updated 2021 Mar 11]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2000 Feb 4 [updated 2021 Mar 11]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301691 Free Books & Documents. Review.
References
-
- Argenta A, Nogueira A, Scheer A (2021) Hydrolysis of whey lactose: Kluyveromyces lactis β-galactosidase immobilisation and integrated process hydrolysis-ultrafiltration. Int Dairy J 117:105007. 10.1016/j.idairyj.2021.105007
-
- Ateş S, Mehmetoğlu Ü (1997) A new method for immobilization of β-galactosidase and its utilization in a plug flow reactor. Process Biochem 32(5):433–436. 10.1016/S0032-9592(96)00101-X
-
- Bouguerra O, Wahab R, Huyop F, Al-Fakih A, Mahmood W, Mahat N, Sabullah M (2024) An overview of crosslinked enzyme aggregates: concept of development and trends of applications. Appl Biochem Biotechnol 196(9):5711–5739. 10.1007/s12010-023-04809-y - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

