Quality of life following ileostomy takedown: single-centre, retrospective clinical trial-does closure time matter?
- PMID: 40736758
- PMCID: PMC12310842
- DOI: 10.1007/s10151-025-03196-2
Quality of life following ileostomy takedown: single-centre, retrospective clinical trial-does closure time matter?
Abstract
Aim: This study aimed to assess whether early closure of loop ileostomy reduces the rate of postoperative complications related to ileostomy closure and improves patients' quality of life, as measured by the Low Anterior Resection Syndrome (LARS) and Wexner questionnaires.
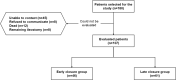
Methods: All patients who underwent low anterior resection + ileostomy with subsequent reversal between January 2019 and May 2023 were included in the study. Patients were divided into two groups: early (< 3 months) and late closure (> 3 months). There were 46 (43%) patients in the early closure group and 61 (57%) in late closure. In this study, patients' demographics and complication rate (categorised by severity using the Clavien-Dindo scale) were assessed.
Results: We assessed and contacted 180 patients. Of these, 107 (59%) completed the LARS and Wexner questionnaires. Of the 107 patients, 51 were male (47.7%) and 56 female (52.3%). The time to ileostomy closure ranged between 0.5 and 28 months, with a median of 5. In the early and late closure groups, postoperative complications were observed in 4.3% vs. 14.8% (p = 0.08) of patients and postoperative ileus occurred in 6.5% vs. 4.9% (p = 0.72) of patients respectively. Median LARS score was 25 vs. 20 (p = 0.99) and Wexner's 2.5 vs. 2 (p = 0.82), respectively. The previously discussed indicators (postoperative ileostomy complications, postoperative ileus rate, LARS and Wexner scores) were not statistically significantly different.
Conclusion: In our small retrospective study, early ileostomy closure did not affect postoperative complications related to ileostomy closure and bowel dysfunction rates compared to late closure.
Trial registration: This study was a secondary analysis of the prospective trial registered at ClinicalTrials.gov no. NCT03607370, 01.07.2017.
Keywords: Loop ileostomy; Low anterior resection syndrome; Oncology; Quality of life; Rectal cancer.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests. Ethical approval: Approval was obtained from Lithuanian Bioethics Committee. The procedures used in this study adhere to the tenets of the Declaration of Helsinki. Consent to participate: Informed consent was obtained from all individual participants included in the study.
Figures
References
-
- Lalinde JD, Caicedo L, Calderon P, Sanchez R, Pareja R (2023) Quality of life in patients undergoing minimally invasive surgery. Int J Gynecol Cancer 33:89–93. 10.1136/ijgc-2022-003931 - PubMed
-
- World Health Organization, Colorectal cancer, 11 July 2023. https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer
-
- Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB (2019) Colorectal cancer. Lancet Lond Engl 394:1467–1480. 10.1016/S0140-6736(19)32319-0 - PubMed
-
- Vogel I, Reeves N, Tanis PJ, Bemelman WA, Torkington J, Hompes R, Cornish JA (2021) Impact of a defunctioning ileostomy and time to stoma closure on bowel function after low anterior resection for rectal cancer: a systematic review and meta-analysis. Tech Coloproctol 25:751–760. 10.1007/s10151-021-02436-5 - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical