Unveiling the common mechanisms and therapeutic targets of medicinal herbs in acute pancreatitis: a network pharmacology and experimental validation approach
- PMID: 40736877
- PMCID: PMC12311076
- DOI: 10.1186/s40643-025-00925-1
Unveiling the common mechanisms and therapeutic targets of medicinal herbs in acute pancreatitis: a network pharmacology and experimental validation approach
Abstract
Background: Acute pancreatitis (AP) is an acute abdominalgia with complicated pathogenesis and high mortality, which is lacking in specific means for clinical diagnosis and treatment. Currently, numerous traditional Chinese medicines have demonstrated remarkable efficacy in AP. Given their multi-target and multi-compound actions, we hypothesize that an underlying common mechanism may contribute to their therapeutic effects. This study aimed to identify key therapeutic targets and potential strategies for AP by investigating the shared pharmacological effects of medicinal plants through network pharmacology analysis and experimental validation.
Methods: We systematically searched the literature for medicinal herbs that have been reported in AP treatment. Next, we utilized the TCMSP database to identify active compounds that were present in at least two medicinal herbs. Key active compounds and targets were determined through Cytoscape analysis and a PPI network, followed by KEGG pathway enrichment analysis. Combined the core targets identified by Cytoscape and the targets enriched in the PI3K/AKT signaling pathway, molecular docking was performed to assess the binding affinity between the intersecting targets and active compounds. Finally, high-affinity compounds were screened, and linarin’s optimal binding profile led to its selection for further in vivo and in vitro experimental validation.
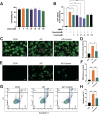
Results: A total of 37 medicinal herbs were retrieved from the literature search. We identified 62 common compounds and 968 targets from medicinal herbs, further taking intersection to 319 targets for anti-AP. Based on this, “compound-target” and “target” networks were constructed, and the top 12 key active compounds and 11 targets were selected. KEGG analysis indicated that the PI3K/AKT pathway might be closely related to pancreatic protection. Molecular docking results showed that linarin exhibited good binding affinity with all core intersecting targets, particularly with AKT1. Subsequently, both in vivo and in vitro experiments demonstrated that linarin could alleviate AP-induced pancreatic damage and systemic inflammation. To further validate the mechanistic involvement of PI3K/AKT signaling pathway, we employed the PI3K/AKT activator 740 Y-P, which was found to effectively reverse linarin-mediated downregulation of PI3K/AKT activation, thereby confirming the crucial role of this pathway in linarin’s protective effects.
Conclusion: Exploring therapeutic strategies based on common mechanisms and targets may be an effective approach. This study revealed that linarin and AKT1 were potential therapeutic compounds and targets for AP in the preclinical stage, which could provide theoretical support and new insights for the drug discovery of AP.
Graphical Abstract:
Supplementary Information: The online version contains supplementary material available at 10.1186/s40643-025-00925-1.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The animal study protocol was approved by the Experimental Animal Ethics Committee, Xiangya Hospital, Central South University, China (approval number: 202503047). Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures





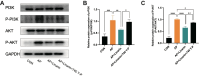
Similar articles
-
Integrating network pharmacology and experimental validation to advance psoriasis treatment: Multi-target mechanistic elucidation of medicinal herbs and natural compounds.Autoimmun Rev. 2025 Jul 31;24(8):103836. doi: 10.1016/j.autrev.2025.103836. Epub 2025 May 15. Autoimmun Rev. 2025. PMID: 40381707
-
Chinese medicinal herbs for acute pancreatitis.Cochrane Database Syst Rev. 2005 Jan 25;2005(1):CD003631. doi: 10.1002/14651858.CD003631.pub2. Cochrane Database Syst Rev. 2005. PMID: 15674909 Free PMC article.
-
Elucidating the Mechanism of Xiaoqinglong Decoction in Chronic Urticaria Treatment: An Integrated Approach of Network Pharmacology, Bioinformatics Analysis, Molecular Docking, and Molecular Dynamics Simulations.Curr Comput Aided Drug Des. 2025 Jul 16. doi: 10.2174/0115734099391401250701045509. Online ahead of print. Curr Comput Aided Drug Des. 2025. PMID: 40676786
-
Medicinal herbs for the treatment of anxiety: A systematic review and network meta-analysis.Pharmacol Res. 2022 May;179:106204. doi: 10.1016/j.phrs.2022.106204. Epub 2022 Apr 1. Pharmacol Res. 2022. PMID: 35378276
-
Network Pharmacology Approach to Unveiling the Mechanism of Wolfberry Mulberry Raspberry Decoction in the Treatment of Sepsis-Induced Myocardial Dysfunction.Drug Des Devel Ther. 2025 Jun 20;19:5293-5310. doi: 10.2147/DDDT.S502301. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40557053 Free PMC article.
References
-
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS Acute pancreatitis classification working, G. (2013) classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut, 62(1), 102–111 - PubMed
-
- Chen X, Zhang S, Xuan Z, Ge D, Chen X, Zhang J, Wang Q, Wu Y, Liu B (2017) The phenolic fraction of mentha Haplocalyx and its constituent Linarin ameliorate inflammatory response through inactivation of NF-kappaB and MAPKs in Lipopolysaccharide-Induced RAW264.7 cells. Molecules, 22(5) - PMC - PubMed
-
- Chen R, Malagola E, Dietrich M, Zuellig R, Tschopp O, Bombardo M, Saponara E, Reding T, Myers S, Hills AP, Graf R, Sonda S (2020) Akt1 signalling supports acinar proliferation and limits acinar-to-ductal metaplasia formation upon induction of acute pancreatitis. J Pathol 250(1):42–54 - PubMed
-
- Chenafa H, Mesli F, Daoud I, Achiri R, Ghalem S, Neghra A (2022) In Silico design of enzyme alpha-amylase and alpha-glucosidase inhibitors using molecular docking, molecular dynamic, conceptual DFT investigation and pharmacophore modelling. J Biomol Struct Dyn 40(14):6308–6329 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

