Exosomal Liquid Biopsy for the Early Detection of Gastric Cancer: The DESTINEX Multicenter Study
- PMID: 40737022
- PMCID: PMC12311825
- DOI: 10.1001/jamasurg.2025.2493
Exosomal Liquid Biopsy for the Early Detection of Gastric Cancer: The DESTINEX Multicenter Study
Abstract
Importance: Gastric cancer (GC) is the third leading cause of cancer-related deaths worldwide, primarily attributed to delayed detection. The invasive and cost-prohibitive nature of endoscopy for GC screening highlights the urgent need for noninvasive biomarkers.
Objective: To develop an exosome-based diagnostic signature to facilitate blood-based, early detection of patients with GC.
Design, setting, and participants: This was a multicenter, population-based, retrospective, case-control study that analyzed specimens collected between January 1, 2016, and December 30, 2020. The study encompassed the discovery, training, validation, and evaluation phases of biomarker development. This study was conducted at 4 major referral centers: Nagoya University Hospital in Japan and Ajou University Hospital, Asan Medical Center, and Samsung Medical Center in South Korea, providing a broad representation of advanced clinical care settings. The study included patients with GC, classified according to the TNM (tumor-node-metastasis) classification (8th edition), and controls without disease. Key sociodemographic data, including age and sex, were recorded for all participants. Data were analyzed from October 2022 to July 2024.
Exposures: Results were obtained from tissue and serum microRNA (miRNA) profiling and expression analysis. Frozen tissue collection and blood draws were conducted intraoperatively, preoperatively, and 3 months postoperatively.
Main outcomes and measures: Diagnostic performance of GC detection using an exosome-based miRNA signature.
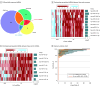
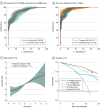
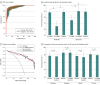
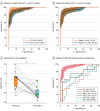
Results: A total of 809 specimens from 480 patients (mean [SD] age, 61.9 [9.8] years; 336 male [70%]) in the training and validation cohorts were analyzed. A panel of 8 cell-free miRNAs and 10 exosomal miRNAs was initially developed in the discovery phase, which was subsequently reduced using machine learning algorithms to a panel of 8 cell-free and 9 exosomal miRNAs during the training phase. This 17-miRNA signature robustly identified GC with area under the curve (AUC) values of 96.3% (95% CI, 94.3%-98.4%) and 95.3% (95% CI, 92.8%-97.9%) in the training and validation cohorts, respectively. Additionally, 5 overlapping miRNAs were observed between cell-free and exosomal panels and exhibited a comparable efficacy in identifying patients with GC. Finally, we established a 10-miRNA signature (Destinex), which successfully identified early-stage (pT1) GC (AUC = 96.8%; 95% CI, 93.5%-100%). Finally, a significant decrease in miRNA expression levels in postsurgery serum specimens confirmed the robustness of the panel specificity.
Conclusion and relevance: Results of this case-control study suggest that the Destinex assay was robust for early detection of GC, highlighting its potential for clinical application in the noninvasive identification of GC.
Conflict of interest statement
Figures
Comment on
-
Liquid Biopsy in Gastric Cancer-Can miRNA Decode a Heterogenous Disease?JAMA Surg. 2025 Sep 1;160(9):983. doi: 10.1001/jamasurg.2025.2471. JAMA Surg. 2025. PMID: 40737014 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

