Use of Pharmacists and Collaborative Practice Agreements to Treat Hepatitis C: A Survey of Primary Care Clinicians in Washington State
- PMID: 40737114
- PMCID: PMC12317179
- DOI: 10.1177/21501319251359547
Use of Pharmacists and Collaborative Practice Agreements to Treat Hepatitis C: A Survey of Primary Care Clinicians in Washington State
Abstract
Background: Collaborative care models that utilize pharmacists can expand hepatitis C (HCV) treatment access, but little is known about primary care provider (PCP) views on such models of care. We characterized PCP experiences of HCV treatment and assessed acceptability of leveraging pharmacists to treat HCV.
Methods: We surveyed a convenience sample of Washington (WA) State PCPs regarding HCV treatment, experience of collaborating with pharmacists, and comfort with pharmacists managing HCV care. We report summarized descriptive statistics of survey responses.
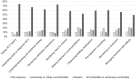
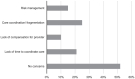
Results: Seventy-three PCPs completed the survey, 55% of whom prescribe buprenorphine for opioid use disorder. Nineteen percent directly treat HCV. Forty-five percent were aware of collaborative practice agreements (CPAs) and 22% reported interest in establishing a CPA to treat HCV. Most respondents were comfortable or extremely comfortable with pharmacists managing key elements of HCV care.
Conclusions: In a sample of WA State PCPs, of whom greater than half prescribe buprenorphine for OUD, fewer than 1 in 5 directly treat HCV. Comfort with pharmacists managing most components of HCV treatment was high, but a minority of PCPs were familiar with or interested in establishing CPAs. Additional efforts are needed to leverage pharmacists to treat HCV, including among people who use drugs.
Keywords: acceptability; collaborative practice agreement; hepatitis C; opioid related disorders; pharmacists.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- CDC. Viral Hepatitis Surveillance- United States, 2018-2022. Accessed October 30, 2024. https://www.cdc.gov/hepatitis/statistics/2022surveillance/hepatitis-c/ta...
-
- World Health Organization. Combating hepatitis B and C to reach elimination by 2030. 2016. World Health Organization; Advocacy Brief. Accessed October 30, 2024. https://iris.who.int/bitstream/handle/10665/206453/WHO_HIV_2016.04_eng.p...
-
- American Association for the Study of Liver Diseases and Infectious Disease Society of America. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. American Association for the Study of Liver Diseases and Infectious Disease Society of America; 2024.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

