Characteristics of medical costs and resource use in patients with rheumatoid arthritis treated with and without glucocorticoids
- PMID: 40737259
- PMCID: PMC12310026
- DOI: 10.1371/journal.pone.0329313
Characteristics of medical costs and resource use in patients with rheumatoid arthritis treated with and without glucocorticoids
Abstract
Objectives: To evaluate medical costs and resource use in patients with rheumatoid arthritis (RA) treated with and without oral or injectable glucocorticoids (GCs) as part of their initial treatment with disease-modifying antirheumatic drugs (DMARDs).
Methods: Patients included in the Japan Medical Data Center health insurance claims database and diagnosed with RA were considered. The date of the first prescription of a DMARD (index date) after an observable 6-month period (baseline) was used to define follow-up (12 months post-index date) periods. Patients with at least one GC prescription in the follow-up period were included in the GC group, and patients without a GC prescription in the follow-up period were classified as the non-GC group. The primary endpoints were costs for drugs, treatments, and materials per patient in the follow-up period. Drugs were divided into medications for RA or for adverse events (AEs). The secondary endpoints were proportions of patients using the subcategories of each resource. The incidence of hospitalization during the follow-up period was evaluated.
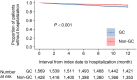
Results: A total of 1,670 and 1,487 patients with median ages of 51.0 and 50.0 years were evaluated in the GC and non-GC groups, respectively. The costs for drugs, treatments, and materials were significantly higher in the GC group compared with the non-GC group (GC/ non-GC; drug costs for RA and AEs, 2,818 USD/ 1,882 USD; drug costs for RA only, 2,697 USD/ 1,805 USD; treatment costs, 2,365 USD/ 1,860 USD; material costs, 112 USD/ 77 USD; P < 0.05). The resource use in almost all drug and treatment subcategories was higher in the GC group. The incidence of hospitalization was also higher in the GC group.
Conclusions: Patients with RA treated with GCs in the first year after starting DMARDs tended to use more resources and have higher medical costs than patients not treated with GCs.
Copyright: © 2025 Tanaka et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
ET has received lecture fees or consulting fees from AbbVie Japan GK, Asahi Kasei Corp., Astellas Pharma Inc., Ayumi Pharmaceutical Co., Boehringer Ingelheim Japan, Inc., Bristol Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi-Sankyo, Inc., Eisai Co., Ltd., Eli Lilly Japan K.K., Gilead Sciences, Inc., Pfizer Japan Inc, Nichi-Iko Pharmaceutical Co., Ltd., Taisho Pharmaceutical Co., Ltd, Takeda Pharmaceutical Co., Ltd, Mitsubishi Tanabe Pharma Co., UCB Japan Co. Ltd. and Viatris Inc. ET has received research funding from Pfizer Inc. and UCB Japan Co. Ltd. EI received lecture fees from Eisai Co., Ltd. and Chugai Pharmaceutical Co., Ltd. RS has nothing to declare. KI was an employee of Medilead, Inc., which was commissioned to perform this study analysis by Tokyo Women's Medical University. During the study, KI was affiliated with Medilead, Inc., but is currently employed by Healthcare Consulting Inc. AS was an employee of Medilead, Inc., which was commissioned to perform this study analysis by Tokyo Women's Medical University. During the study, KI was affiliated with Medilead, Inc., but is currently employed by Healthcare Consulting Inc and the University of Tokyo. MH has received research grants from AbbVie Japan GK, Asahi Kasei Corp., Ayumi Pharmaceutical Co., Boehringer Ingelheim Japan, Inc., Bristol Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Eisai Co., Ltd., Eli Lilly Japan K.K., Kaken Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co., Mochida Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Pfizer Japan Inc., Taisho Pharmaceutical Co., Ltd., Teijin Pharma Ltd., UCB Japan Co., Ltd., and Viatris Japan. MH has received speaker’s fee from AbbVie Japan GK, Asahi Kasei Corp., Astra Zeneca K. K., Ayumi Pharmaceutical Co., Boehringer Ingelheim Japan, Inc., Bristol Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan K.K., GlaxoSmithKline K.K., Gilead Sciences Inc., Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Co., Mochida Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Pfizer Japan Inc., Taisho Pharmaceutical Co., Ltd., and Teijin Pharma Ltd. MH is a consultant for AbbVie, Boehringer-ingelheim, Bristol Myers Squibb Co., and Teijin Pharma.
Figures

Similar articles
-
Medical costs for patients with rheumatoid arthritis who have comorbid diabetes mellitus.PLoS One. 2025 Aug 1;20(8):e0328094. doi: 10.1371/journal.pone.0328094. eCollection 2025. PLoS One. 2025. PMID: 40749000 Free PMC article.
-
A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness.Health Technol Assess. 2006 Nov;10(42):iii-iv, xi-xiii, 1-229. doi: 10.3310/hta10420. Health Technol Assess. 2006. PMID: 17049139
-
The clinical and cost-effectiveness of anakinra for the treatment of rheumatoid arthritis in adults: a systematic review and economic analysis.Health Technol Assess. 2004 May;8(18):iii-iv, ix-x, 1-105. doi: 10.3310/hta8180. Health Technol Assess. 2004. PMID: 15130461
-
Glucocorticoid use in rheumatoid arthritis patients and the onset of pneumonia: a systematic review and meta-analysis.J Osteopath Med. 2023 Jan 25;123(4):179-186. doi: 10.1515/jom-2022-0177. eCollection 2023 Apr 1. J Osteopath Med. 2023. PMID: 36691851
-
Including adverse drug events in economic evaluations of anti-tumour necrosis factor-α drugs for adult rheumatoid arthritis: a systematic review of economic decision analytic models.Pharmacoeconomics. 2014 Feb;32(2):109-34. doi: 10.1007/s40273-013-0120-z. Pharmacoeconomics. 2014. PMID: 24338344
References
-
- Smolen JS, Landewé RBM, Bergstra SA, Kerschbaumer A, Sepriano A, Aletaha D, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023;82:3–18. - PubMed
-
- Kawahito Y, Morinobu A, Kaneko Y, Kohno M, Hirata S, Kishimoto M, et al. Drug treatment algorithm and recommendations from the 2020 update of the Japan College of Rheumatology clinical practice guidelines for the management of rheumatoid arthritis-secondary publication. Mod Rheumatol. 2023;33(1):21–35. doi: 10.1093/mr/roac017 - DOI - PubMed
-
- Santiago T, da Silva JA. Safety of low- to medium-dose glucocorticoid treatment in rheumatoid arthritis: myths and reality over the years. Ann N Y Acad Sci. 2014;1318:41–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

