Anti-Cryptosporidium efficacy of BKI-1708, an inhibitor of Cryptosporidium calcium-dependent protein kinase 1
- PMID: 40737275
- PMCID: PMC12310023
- DOI: 10.1371/journal.pntd.0013263
Anti-Cryptosporidium efficacy of BKI-1708, an inhibitor of Cryptosporidium calcium-dependent protein kinase 1
Abstract
Background: Diarrheal pathogens, such as Cryptosporidium, impose a heavy burden of disease in resource-limited regions. Cryptosporidiosis often causes chronic infection in immunocompromised people and gastrointestinal injury in malnourished children, leading to wasting, stunting, and cognitive impairment. Current treatment for cryptosporidiosis fails in these vulnerable populations, highlighting the need for new medicines. Here we describe the anti-Cryptosporidium efficacy, pharmacokinetics, and safety of a bumped kinase inhibitor BKI-1708. BKI-1708 inhibits the essential molecular target, calcium-dependent protein kinase 1 (CDPK1), which is highly expressed in the major proliferative stages of the parasite life cycle.
Methods and findings: Efficacy was demonstrated in the Cryptosporidium parvum IFNγ-KO mouse infection and calf diarrhea models. Dose response in the mouse model demonstrated oral doses as low as 15 mg/kg administered daily for 3 days completely suppressed oocyst shedding. Metabolite profiling in pre-clinical species and human hepatocytes identified an active metabolite, M2, which retains sub-micromolar activity against C. parvum. Pharmacokinetic analysis of BKI-1708 and M2 in mice demonstrates good systemic exposure, important for treating biliary and upper respiratory infections in some cases of cryptosporidiosis. In mice, M2 reaches 7-fold and >3-fold higher levels over BKI-1708 in plasma and the gastrointestinal tract, respectively. Oral administration of M2 completely suppressed oocyst shedding in the mouse model at doses as low as 8 mg/kg for 3 days. Wide safety margins are demonstrated in mice, rats, and dogs.
Conclusions: BKI-1708 has characteristics of a safe and effective drug for treating Cryptosporidium infections in animal models and shows promise for use in humans. Moreover, BKI-1708 and M2 formed in vivo, offer an attractive prospect of a dually active preclinical candidate for the treatment of cryptosporidiosis.
Copyright: © 2025 Choi et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: W.C.V.V. is the president of ParaTheraTech Inc, a company involved with developing BKIs for use in animal health. The following authors are members of an advocacy group, called "Cryptosporidiosis Therapeutics Advocacy Group" or CTAG: R.C., M.A.H., D.A.S., D.P.B., M.W.R., S.L.M.A., L.K.B., K.K.O., E.F., and W.C.V.V. CTAG is advocating to increase knowledge of the human toll of Cryptosporidiosis, to add Cryptosporidiosis to the Neglected Tropical Diseases list at the WHO, and to the US FDA Priority Review Voucher program. These competing interests will not alter adherence to PLOS policies on sharing data and materials. All other authors declare that they hold no competing interests.
Figures



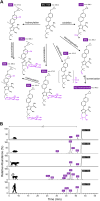
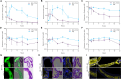
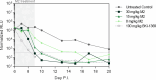
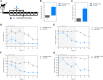
References
-
- Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388(10063):3027–35. doi: 10.1016/S0140-6736(16)31593-8 - DOI - PMC - PubMed
-
- GBD Diarrhoeal Diseases Collaborators. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17(9):909–48. doi: 10.1016/S1473-3099(17)30276-1 - DOI - PMC - PubMed
-
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–22. doi: 10.1016/S0140-6736(13)60844-2 - DOI - PubMed
-
- Sow SO, Muhsen K, Nasrin D, Blackwelder WC, Wu Y, Farag TH, et al. The Burden of Cryptosporidium Diarrheal Disease among Children < 24 Months of Age in Moderate/High Mortality Regions of Sub-Saharan Africa and South Asia, Utilizing Data from the Global Enteric Multicenter Study (GEMS). PLoS Negl Trop Dis. 2016;10(5):e0004729. doi: 10.1371/journal.pntd.0004729 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

