Role of rosuvastatin and pitavastatin in alleviating diabetic cardiomyopathy in rats: Targeting of RISK, NF-κB/ NLRP3 inflammasome and TLR4/ NF-κB signaling cascades
- PMID: 40737341
- PMCID: PMC12310044
- DOI: 10.1371/journal.pone.0325767
Role of rosuvastatin and pitavastatin in alleviating diabetic cardiomyopathy in rats: Targeting of RISK, NF-κB/ NLRP3 inflammasome and TLR4/ NF-κB signaling cascades
Abstract
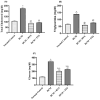
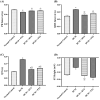
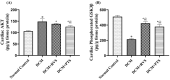
Diabetic cardiomyopathy (DCM) is a serious outcome of type II diabetes mellitus (T2DM) and a key contributor to high morbidity and death in diabetic individuals. The current research is intended to elucidate and compare the therapeutic benefits of rosuvastatin (RVS) and pitavastatin (PTS) in mitigating DMC-induced in rats and exploring the possible underlying molecular signaling pathways. DCM was prompted by feeding rats a high-fat/fructose (F/Fr) diet for eight weeks with a sub-diabetogenic dose of streptozotocin (35 mg/kg; i.p) injection at week seven. All rats were allocated into four groups: a normal control group, a DCM-induced positive control group, the RVS group of DCM-induced rats that were treated once daily with 10 mg/kg of RVS, and the PTS group of DCM rats that were treated with 0.8 mg/kg of PTS. Rats were given the treatments orally for four consecutive weeks. The outcome of the existing work discovered that RVS and PTS significantly improved T2DM-associated DCM, as evidenced by the amelioration of glucose, lipids, cardiac markers, ECG parameters, and redox status. Considering the relationship between oxidative stress and inflammation, this attenuation was evidenced by the downregulation of redox, inflammatory, and cellular fibrotic cascades, namely RISK, NF-κB/NLRP3 inflammasome, and TLR4/NF-κB signaling pathways. Additionally, the histopathological examinations confirmed these structural alterations in the myocardium. Besides, RVS and PTS diminished the expression of caspase-1 assessed by immunochemical staining. In summary, the present study demonstrated that RVS and PTS mitigated the metabolic abnormalities associated with T2DM-induced DCM.
Copyright: © 2025 Saleh et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

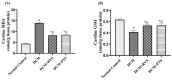








Similar articles
-
Astaxanthin mitigates diabetic cardiomyopathy and nephropathyin HF/HFr/STZ diabetic rats via modulating NOX4, fractalkine, Nrf2, and AP-1 pathways.Sci Rep. 2025 Jun 20;15(1):20199. doi: 10.1038/s41598-025-06263-8. Sci Rep. 2025. PMID: 40542047 Free PMC article.
-
Chronic Anatabine Administration Attenuates Cardiovascular Activity by Targeting NF-κB/NLRP3/Caspase-1-Dependent Pyroptosis and Oxidative Stress in Paraventricular Nucleus of Hypertensive Rat.Cardiovasc Toxicol. 2025 Sep;25(9):1352-1368. doi: 10.1007/s12012-025-10034-2. Epub 2025 Jul 21. Cardiovasc Toxicol. 2025. PMID: 40690148
-
Modulation of glycolytic metabolic reprogramming and the TLR4/NF-κB/NLRP3 Pathway: comparative analysis of anti-inflammatory activity between two dosage forms of Pudilan xiaoyan.J Ethnopharmacol. 2025 Aug 29;352:120204. doi: 10.1016/j.jep.2025.120204. Epub 2025 Jun 24. J Ethnopharmacol. 2025. PMID: 40571230
-
NTP Developmental and Reproductive Toxicity Technical Report on the Prenatal Development Studies of 2-((1-(4-Phenoxyphenoxy)propan-2-yl)oxy)pyridine (CASRN 95737-68-1) in Sprague Dawley (Hsd:Sprague Dawley® SD®) Rats and New Zealand White (Hra:NZW SPF) Rabbits: DART Report 07 [Internet].Research Triangle Park (NC): National Toxicology Program; 2022 Jan. Research Triangle Park (NC): National Toxicology Program; 2022 Jan. PMID: 35593777 Free Books & Documents. Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Hou J, Zheng D, Fung G, Deng H, Chen L, Liang J, et al. Mangiferin suppressed advanced glycation end products (AGEs) through NF-κB deactivation and displayed anti-inflammatory effects in streptozotocin and high fat diet-diabetic cardiomyopathy rats. Can J Physiol Pharmacol. 2016;94(3):332–40. doi: 10.1139/cjpp-2015-0073 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

