Machine learning-assisted exploration of multidrug-drug administration regimens for organoid arrays
- PMID: 40737392
- PMCID: PMC12309667
- DOI: 10.1126/sciadv.adt1851
Machine learning-assisted exploration of multidrug-drug administration regimens for organoid arrays
Abstract
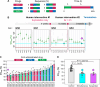
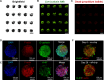
Combination therapies enhance the therapeutic effect of cancer treatment; however, identifying effective interdependent doses, durations, and sequences of multidrug administration regimens is a time- and labor-intensive task. Here, we integrated machine learning, automation, and large microfluidic arrays of cancer spheroids or patient-derived organoids formed in a tissue-mimetic hydrogel to achieve notable acceleration of the discovery of effective multidrug administration regimens. For the clinically approved drug combination, we found a sequential administration regimen leading to a substantial reduction in the total drug dose, in comparison with concurrent drug supply, both at comparable drug efficacy. For the drugs that are currently under clinical development, we found a synergistic effect of concurrently administered drugs and showed that the synergy diminishes for the sequential drug supply. The developed strategy holds promise for the discovery of effective combination therapies for advanced cancer treatment, including personalized chemotherapies.
Figures





Similar articles
-
Chemotherapy for advanced gastric cancer.Cochrane Database Syst Rev. 2017 Aug 29;8(8):CD004064. doi: 10.1002/14651858.CD004064.pub4. Cochrane Database Syst Rev. 2017. PMID: 28850174 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Organoid Models Established from Primary Tumors and Patient-Derived Xenograft Tumors Reflect Platinum Sensitivity of Ovarian Cancer Patients.bioRxiv [Preprint]. 2025 May 2:2024.06.28.601283. doi: 10.1101/2024.06.28.601283. bioRxiv. 2025. PMID: 40654830 Free PMC article. Preprint.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2011 Jul 6;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. Cochrane Database Syst Rev. 2011. PMID: 21735394
References
-
- Harbeck N., Penault-Llorca F., Cortes J., Gnant M., Houssami N., Poortmans P., Ruddy K., Tsang J., Cardoso F., Breast cancer. Nat. Rev. Dis. Primers 5, 66 (2019). - PubMed
-
- Telli M. L., Carlson R. W., First-line chemotherapy for metastatic breast cancer. Clin. Breast Cancer 9, S66–S72 (2009). - PubMed
-
- Hortobagyi G. N., Buzdar A. U., Current status of adjuvant systemic therapy for primary breast cancer: Progress and controversy. CA Cancer J. Clin. 45, 199–226 (1995). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

