A trehalase-derived MAMP triggers LecRK-V-mediated immune responses in Arabidopsis
- PMID: 40737394
- PMCID: PMC12309661
- DOI: 10.1126/sciadv.adv8896
A trehalase-derived MAMP triggers LecRK-V-mediated immune responses in Arabidopsis
Abstract
Plant-parasitic nematodes (PPNs) cause major agricultural losses worldwide, yet the molecular basis of plant immunity against these pathogens remains poorly understood. To investigate how plants recognize PPNs, we aimed to identify microbe-associated molecular patterns (MAMPs) from nematodes and the corresponding plant immune components. Because of the limited availability of material from obligate PPNs, we used Caenorhabditis elegans, a free-living nematode, as a MAMP source. C. elegans extract activated MAMP-triggered immune responses in Arabidopsis Col-0. Through chromatography-based purification, we identified a secreted trehalase and pinpointed a conserved peptide region essential for its MAMP activity. A corresponding peptide from root-knot nematode trehalase enabled the identification of lectin receptor kinases LecRK-V.5 and LecRK-V.6 as key components in immune induction. Notably, this peptide region is conserved across some phytophagous insects and fungal pathogens, with LecRK-Vs required for immune responses to these peptides, highlighting the role of LecRK-V-mediated mechanism for broad-spectrum pathogen detection via trehalase-derived peptides.
Figures


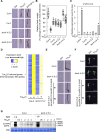

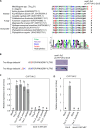
References
-
- M. R. Khan, in Novel Biological and Biotechnological Applications in Plant Nematode Management, M. R. Khan, Ed. (Springer Nature Singapore, Singapore, 2023), pp. 3–45.
-
- Molloy B., Baum T., Eves-van den Akker S., Unlocking the development- and physiology-altering ‘effector toolbox’ of plant-parasitic nematodes. Trends Parasitol. 39, 732–738 (2023). - PubMed
-
- Siddique S., Grundler F. M., Parasitic nematodes manipulate plant development to establish feeding sites. Curr. Opin. Microbiol. 46, 102–108 (2018). - PubMed
-
- Couto D., Zipfel C., Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 16, 537–552 (2016). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

