First Decade of the National Cancer Institute's Affordable Cancer Technologies Program: Accelerating Translational Technology Research and Development for Cancer Globally
- PMID: 40737580
- PMCID: PMC12320939
- DOI: 10.1200/GO-25-00200
First Decade of the National Cancer Institute's Affordable Cancer Technologies Program: Accelerating Translational Technology Research and Development for Cancer Globally
Abstract
Since 2014, the National Cancer Institute Affordable Cancer Technologies (ACTs) program has supported a broad research portfolio focused on the development and validation of new technologies for global cancer control. ACTs projects are conducted by international teams composed of investigators from the United States and low- and middle-income countries, spurring important contextually relevant innovations. During its first decade, the ACTs program ushered in new technology platforms, led to commercialized products, and affected health policies and programs worldwide including in the United States. It has allowed a new generation of investigators working across disciplines and national borders to pursue novel technological solutions and leverage new analytic methods to advance human health. This commentary lays out the scope and accomplishments of the ACTs program to date while considering possible future research directions.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No potential conflicts of interest were reported.
Figures
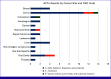
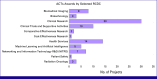
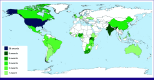
References
-
- Pearlman PC, Divi R, Gwede M, et al. The National Institutes of Health affordable cancer technologies program: Improving access to resource-appropriate technologies for cancer detection, diagnosis, monitoring, and treatment in low- and middle-income countries. IEEE J Transl Eng Health Med. 2016;4:2800708. - PMC - PubMed
-
- Varmus H, Trimble EL. Integrating cancer control into global health. Sci Transl Med. 2011;3:101cm28. - PubMed
-
- National Institutes of Health, Fogarty International Center . Mobile Health Technology and Outcomes in LMICs (R21 R33) Fogarty International Center; n.d.. https://www.fic.nih.gov/Programs/Pages/mhealth.aspx
-
- Digital Science . Dimensions. 2018. https://app.dimensions.ai under licence agreement.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

