Leveraging Interoperable Electronic Health Record (EHR) Data for Distributed Analyses in Clinical Research: Technical Implementation Report of the HELP Study
- PMID: 40737605
- PMCID: PMC12310147
- DOI: 10.2196/68171
Leveraging Interoperable Electronic Health Record (EHR) Data for Distributed Analyses in Clinical Research: Technical Implementation Report of the HELP Study
Abstract
Background: The Medical Informatics Initiative (MII) Germany established 38 data integration centers (DIC) in university hospitals to improve health care and biomedical research through the use of electronic health record (EHR) data. To showcase the value of these DIC, the HELP (Hospital-wide Electronic Medical Record Evaluated Computerized Decision Support System to Improve Outcomes of Patients with Staphylococcal Bloodstream Infection) study was initiated as a use case. This study is a clinical trial designed to assess the impact of a computerized decision support system for managing staphylococcal bacteremia.
Objective: In this paper, we present the lessons learned during the use case from a technical perspective. This paper outlines the challenges encountered and solutions developed during our initial implementation of this infrastructure, providing insights applicable to other research platforms using EHR data. These insights are organized into 3 key areas: study-specific data definition and modeling, interoperable data integration and transformation, and distributed data extraction and analysis.
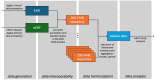
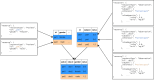
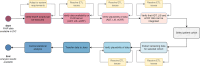
Methods: An interdisciplinary team of clinicians, computer scientists, and statisticians created a catalog of items to identify data elements necessary for the study's evaluation and developed a domain-specific information model. DIC developed extract-transform-load pipelines to collect the disparate, site-specific EHR data and to transform it into a common data format. Health Level Seven International (HL7) Fast Healthcare Interoperability Resources (FHIR) and the MII's core dataset profiles were adopted for consistent data representation across sites. Additionally, data not present in EHRs was gathered using structured electronic case report forms. Analysis scripts were then distributed to the sites to preprocess the data locally, followed by a central analysis of the preprocessed data to generate the final overall results.
Our analysis revealed significant heterogeneity in data quality and implementation of interoperability standards, requiring substantial harmonization efforts. The development of analysis scripts and data extraction processes demanded multiple iterative cycles and close collaboration with local data experts. Despite these challenges, the successful implementation demonstrated the feasibility of distributed EHR analyses while highlighting the importance of thorough data quality assessment, realistic timeline planning, and multidisciplinary expertise.
Conclusions: The HELP study highlights challenges and opportunities in leveraging EHR data for clinical research, particularly in the absence of mandatory data standards and resource-intensive data harmonization efforts. Despite limitations in data availability and quality, progress in digitization and interoperability frameworks offers hope for future improvements. Lessons learned from this study can inform the development of standardized methodologies and infrastructures for sustainable EHR data integration in research.
Keywords: clinical decision support system; data collection methods; electronic health records; health information interoperability; software design.
© Julia Palm, Kutaiba Saleh, André Scherag, Danny Ammon. Originally published in JMIR Medical Informatics (https://medinform.jmir.org).
Conflict of interest statement
Figures


Similar articles
-
Accreditation through the eyes of nurse managers: an infinite staircase or a phenomenon that evaporates like water.J Health Organ Manag. 2025 Jun 30. doi: 10.1108/JHOM-01-2025-0029. Online ahead of print. J Health Organ Manag. 2025. PMID: 40574247
-
Minimal Common Oncology Data Elements Genomics Pilot Project: Enhancing Oncology Research Through Electronic Health Record Interoperability at Vanderbilt University Medical Center.JCO Clin Cancer Inform. 2024 Jun;8:e2300249. doi: 10.1200/CCI.23.00249. JCO Clin Cancer Inform. 2024. PMID: 38935887 Free PMC article.
-
Bridging Global Frameworks and Local Practice: Quantitative Evaluation of Electronic Health Record Safety in Kuwait's Public Hospitals.JMIR Med Inform. 2025 Aug 14;13:e70782. doi: 10.2196/70782. JMIR Med Inform. 2025. PMID: 40811701
-
Electronic Health Record and Semantic Issues Using Fast Healthcare Interoperability Resources: Systematic Mapping Review.J Med Internet Res. 2024 Jan 30;26:e45209. doi: 10.2196/45209. J Med Internet Res. 2024. PMID: 38289660 Free PMC article.
-
Designing Clinical Decision Support Systems (CDSS)-A User-Centered Lens of the Design Characteristics, Challenges, and Implications: Systematic Review.J Med Internet Res. 2025 Jun 20;27:e63733. doi: 10.2196/63733. J Med Internet Res. 2025. PMID: 40540451 Review.
References
-
- Albashiti F, Thasler R, Wendt T, Bathelt F, Reinecke I, Schreiweis B. Data integration centers-from a concept in the Medical Informatics Initiative to its local implementation in the Network of University Medicine. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2024 Jun;67(6):629–636. doi: 10.1007/s00103-024-03879-5. doi. Medline. - DOI - PMC - PubMed
-
- Hagel S, Gantner J, Spreckelsen C, et al. Hospital-wide ELectronic medical record evaluated computerised decision support system to improve outcomes of Patients with staphylococcal bloodstream infection (HELP): study protocol for a multicentre stepped-wedge cluster randomised trial. BMJ Open. 2020 Feb 10;10(2):e033391. doi: 10.1136/bmjopen-2019-033391. doi. Medline. - DOI - PMC - PubMed
-
- Pletz M, Hagel S, Kimmig A, et al. Zenodo; 2024. HELP CDSS v. 1.0.SMITH - smart medical information technology for healthcare.https://zenodo.org/records/10704513 URL. doi. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources

