The association between circulating SIGLEC6 and preeclampsia: observational studies of seven cohorts
- PMID: 40737757
- PMCID: PMC12320669
- DOI: 10.1016/j.ebiom.2025.105870
The association between circulating SIGLEC6 and preeclampsia: observational studies of seven cohorts
Abstract
Background: Preeclampsia is a serious complication of pregnancy.
Methods: We did an observational study using seven tissue bank/cohorts to examine the association between circulating SIGLEC6 and preeclampsia. We included samples from participants with preterm disease (delivering <34 weeks gestation in Australia), examined whether levels altered with clinical disease severity (samples collected in South Africa) and whether there were alterations preceding disease onset using samples collected at 15- and 20-weeks' gestation in New Zealand, samples collected between 26 and 34 weeks in the UK and samples collected at 28 or 36 weeks gestation in Australia. Circulating SIGLEC6, sFlt-1, and PlGF were measured via ELISA or a electrochemiluminescence immunoassay platform.
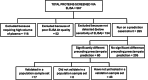
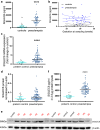
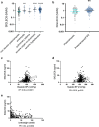
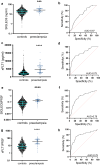
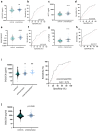
Findings: SIGLEC6 was elevated 9.5-fold (23,397 pg/ml, IQR 16701-32,267) in preterm preeclampsia (<34 weeks gestation), compared to normotensive pregnancies (2441 pg/ml, IQR 871.9-6547; p = 6.3 × 10-9). SIGLEC6 levels correlated with disease severity: compared to preeclampsia without severe features, SIGLEC6 was raised 1.5-2.5-fold with eclampsia, or preeclampsia with life-threatening complications. There was a stepwise increase in SIGLEC6 with increasing numbers of maternal complications, accentuated when expressed as a SIGLEC6/PlGF ratio (10.7-fold rise with ≥3 maternal complications, versus no complications). Circulating SIGLEC6 concentrations were significantly increased among those later diagnosed with preeclampsia in samples collected at 36 weeks (n = 1032; Australia), 26-34 weeks (n = 235; UK), 28 (n = 283; Australia), and 20 weeks' gestation (n = 1945; New Zealand).
Interpretation: SIGLEC6 is elevated with preeclampsia and levels correlate with disease severity.
Funding: National Health and Medical Research Council (#1065854) and the Norman Beischer Medical Research Foundation. Additional sources of funding for the biobank from South Africa was received from the Swedish Medical Society, Märta Lundqvist Foundation, Swedish Foundation for International Cooperation in Research and Higher Education, Jane and Dan Olssons Foundation, Mercy Perinatal (Australia), the Swedish Research Council (Vetenskaps-rådet), Sweden, and the Center for Clinical Research Dalarna, Sweden. The MAViS study (UK) was funded through National Institute Health Research (NIHR-CS-011-020). MUMS was funded by a St George and Sutherland Medical Research Foundation of Australia grant. Salary or scholarship support was received from: Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG) Taylor Hammond Scholarship to TM; National Health and Medical Research Council Fellowships to ST (#2017897) and DMK (#2008017); Australian Research Council Future Fellowships to TKL (FT230100125) and NJH (FT210100193), Senior Medical Research Fellowship from the Sylvia and Charles Viertel Charitable Foundation Fellowship and a National Heart Foundation Future Leader Fellowship (#105663) to FZM.
Keywords: Biomarker; Placenta; Preeclampsia; Pregnancy; SIGLEC6.
Copyright © 2025 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests ST declares a relationship with Diamedica Therapeutics, receiving consultancy payments to develop an investigational drug unrelated to this current project. All other authors have no conflicts of interest to declare.
Figures
References
-
- Chappell L.C., Cluver C.A., Kingdom J., Tong S. Pre-eclampsia. Lancet. 2021;398(10297):341–354. - PubMed
-
- Chappell L.C., Duckworth S., Seed P.T., et al. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: a prospective multicenter study. Circulation. 2013;128(19):2121–2131. - PubMed
-
- Whigham C.A., MacDonald T.M., Walker S.P., Hannan N.J., Tong S., Kaitu'u-Lino T.J. The untapped potential of placenta-enriched molecules for diagnostic and therapeutic development. Placenta. 2019;84:28–31. - PubMed
-
- BioGPS Gene portal system. 2014. www.biopgs.org
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

