Metagenomic selections reveal diverse antiphage defenses in human and environmental microbiomes
- PMID: 40738105
- PMCID: PMC12366544
- DOI: 10.1016/j.chom.2025.07.005
Metagenomic selections reveal diverse antiphage defenses in human and environmental microbiomes
Abstract
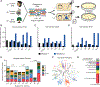
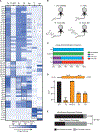
To prevent phage infection, bacteria have developed an arsenal of antiphage defenses. Evidence suggests that many examples in nature have not been described. Using plasmid libraries expressing small DNA inserts and functional selections for antiphage defense in Escherichia coli, we identified over 200 putative defenses from 14 bacterial phyla in 9 human and soil microbiomes. Many defenses were unrecognizable based on sequence or predicted structure and thus could only be identified via functional assays. In mechanistic studies, we show that some defenses encode nucleases that distinguish phage DNA via diverse chemical modifications. We also identify outer membrane proteins that prevent phage adsorption and a set of unknown defenses with diverse antiphage profiles and modalities. Most defenses acted against at least two phages, indicating that broadly acting systems are widely distributed. Collectively, these findings highlight the diversity and interoperability of antiphage defense systems.
Keywords: functional metagenomics; genomics; horizontal gene transfer; metagenomics; microbial; nuclease; phage; phage biology; phage defense; phylogenetics.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare that they have no competing interests.
Figures



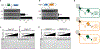
Update of
-
Metagenomic selections reveal diverse antiphage defenses in human and environmental microbiomes.bioRxiv [Preprint]. 2025 Mar 1:2025.02.28.640651. doi: 10.1101/2025.02.28.640651. bioRxiv. 2025. Update in: Cell Host Microbe. 2025 Aug 13;33(8):1381-1395.e7. doi: 10.1016/j.chom.2025.07.005. PMID: 40060627 Free PMC article. Updated. Preprint.
Similar articles
-
Metagenomic selections reveal diverse antiphage defenses in human and environmental microbiomes.bioRxiv [Preprint]. 2025 Mar 1:2025.02.28.640651. doi: 10.1101/2025.02.28.640651. bioRxiv. 2025. Update in: Cell Host Microbe. 2025 Aug 13;33(8):1381-1395.e7. doi: 10.1016/j.chom.2025.07.005. PMID: 40060627 Free PMC article. Updated. Preprint.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Horizontal Gene Transfer and CRISPR Targeting Drive Phage-Bacterial Host Interactions and Coevolution in "Pink Berry" Marine Microbial Aggregates.Appl Environ Microbiol. 2023 Jul 26;89(7):e0017723. doi: 10.1128/aem.00177-23. Epub 2023 Jul 5. Appl Environ Microbiol. 2023. PMID: 37404190 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Factors that influence participation in physical activity for people with bipolar disorder: a synthesis of qualitative evidence.Cochrane Database Syst Rev. 2024 Jun 4;6(6):CD013557. doi: 10.1002/14651858.CD013557.pub2. Cochrane Database Syst Rev. 2024. PMID: 38837220 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

