Metabolic Profile Associated With Encystation in Acanthamoeba
- PMID: 40738523
- PMCID: PMC12310340
- DOI: 10.1111/jeu.70034
Metabolic Profile Associated With Encystation in Acanthamoeba
Abstract
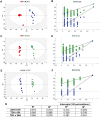
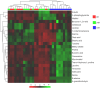
The genus Acanthamoeba includes widespread protozoa that can cause severe infections in humans. Their ability to form resistant cysts within infected tissues complicates treatment, making it essential to understand the encystation process for developing effective therapeutic strategies. This study utilized untargeted metabolomics (GC-MS) to analyze metabolic changes during the encystation of an Acanthamoeba strain in Neff's encystation saline. We conducted metabolite analysis at three stages of differentiation: the trophozoite-dominant phase (0 h), the pre-cyst-dominant phase (24 h), and the cyst-dominant phase (72 h). The results indicated a global metabolic downregulation during encystation, which is consistent with a state of dormancy. Components of the cyst wall such as cellobiose and lactose accumulated in the final phase. Arbutin and canavanine were annotated for the first time in Acanthamoeba. Encystation also led to changes in pathways related to glycine, serine, and threonine metabolism and biosynthesis of aminoacyl-tRNA. This study uncovered previously unknown metabolites and metabolic pathways at distinct stages of Acanthamoeba development.
Keywords: acanthamoeba; encystation; gas chromatography–mass spectrometry; metabolomics.
© 2025 The Author(s). Journal of Eukaryotic Microbiology published by Wiley Periodicals LLC on behalf of International Society of Protistologists.
Figures


References
-
- Aksozek, A. , McClellan K., Howard K., Niederkorn J. Y., and Alizadeh H.. 2002. “Resistance of <styled-content style="fixed-case"> Acanthamoeba castellanii </styled-content> Cysts to Physical, Chemical, and Radiological Conditions.” Journal of Parasitology 88: 621–623. 10.1645/0022-3395(2002)088[0621:ROACCT]2.0.CO;2. - DOI - PubMed
-
- Bauer, H. 1967. “Ultrastruktur und Zellwandbildung von Acanthamoeba sp.” Vierteljahrsschrift Der Naturforschenden Gesellschaft in Zürich 112: 173–197.
-
- Benedetto, N. , Rossano F., Gorga F., Folgore A., Rao M., and Romano Carratelli C.. 2003. “Defense Mechanisms of IFN‐Gamma and LPS‐Primed Murine Microglia Against <styled-content style="fixed-case"> Acanthamoeba castellanii </styled-content> Infection.” International Immunopharmacology 3: 825–834. 10.1016/S1567-5769(03)00047-X. - DOI - PubMed
MeSH terms
Grants and funding
- BPD-00352-22/Fundação de Amparo à Pesquisa do Estado de Minas Gerais
- red001214 via Rede Mineira de Biomoléculas/Fundação de Amparo à Pesquisa do Estado de Minas Gerais
- 573547/2008-4/Conselho Nacional de Desenvolvimento Científico e Tecnológico
- 465293/2014-0 via INCTv/Conselho Nacional de Desenvolvimento Científico e Tecnológico
- 467640/2014-9/Conselho Nacional de Desenvolvimento Científico e Tecnológico
LinkOut - more resources
Full Text Sources
Miscellaneous

