Virus-like particle vaccines targeting a key epitope in circumsporozoite protein provide sterilizing immunity against malaria
- PMID: 40738902
- PMCID: PMC12311073
- DOI: 10.1038/s41541-025-01241-7
Virus-like particle vaccines targeting a key epitope in circumsporozoite protein provide sterilizing immunity against malaria
Abstract
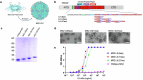
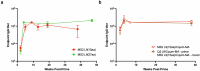
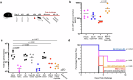
Vaccines that target the pre-erythrocytic stage of the malaria lifecycle have the potential to provide sterilizing immunity but must elicit sustained, high-titer antibody responses to completely prevent infection. Most pre-erythrocytic vaccines target circumsporozoite protein (CSP), the major surface antigen on Plasmodium falciparum sporozoites. Antibodies targeting distinct epitopes within the central repeat region of CSP have the potential to provide protection from infection, but we have focused on developing vaccines that target a highly vulnerable CSP epitope that is targeted by the potent monoclonal antibody L9. In a previous study, we produced a pre-erythrocytic vaccine displaying a synthetic peptide representing the L9 epitope on Qβ bacteriophage virus-like particles (VLPs). This vaccine elicited strong anti-CSP antibody responses that protected mice from malaria challenge. Here, we asked whether the structural context of the L9 epitope influences the quality of antibody responses. We compared the immunogenicity and protective efficacy of Qβ L9 VLPs to recombinant VLPs that display the L9 peptide in a structure that is hypothesized to mimic its native conformation. Recombinant MS2 bacteriophage VLPs displaying various lengths of the L9 epitope were produced and immunogenicity and protective efficacy were evaluated in mice. Our results demonstrate that MS2 L9 VLPs, particularly those displaying longer L9 peptides and in combination with a potent novel adjuvant, elicit strong and durable antibody responses that lower malaria liver burden and prevent infection. We also compared the efficacy of L9-targeted vaccines to the licensed vaccine, RTS,S/AS01E (Mosquirix™, GSK). Immunization with Qβ L9 VLPs, MS2 L9 VLPs, and RTS,S/AS01E provided significant protection from liver-stage infection in a mouse model. Interestingly, immunization with a combination vaccine consisting of MS2 L9 and Qβ L9 VLPs, each presenting the L9 epitope in a distinct structural context, elicited sterilizing immunity in the highest percentage of mice.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: B.C. has equity in Metaphore Biotechnologies. S.A.D. is affiliated with ViroVax LLC, which holds proprietary rights over the Cquim-MA adjuvant. F.Z. serves as an Associate Editor of this journal and had no role in the peer-review or decision to publish this manuscript. F.Z. declares no financial competing interests. All other authors (Y.N., A.F., Y.F-G., and B.T.R.) declare no competing financial or non-financial competing interests.
Figures


Update of
-
Virus-like particle vaccines targeting a key epitope in circumsporozoite protein provide sterilizing immunity against malaria.bioRxiv [Preprint]. 2025 May 30:2025.03.03.641239. doi: 10.1101/2025.03.03.641239. bioRxiv. 2025. Update in: NPJ Vaccines. 2025 Jul 30;10(1):176. doi: 10.1038/s41541-025-01241-7. PMID: 40463147 Free PMC article. Updated. Preprint.
Similar articles
-
Virus-like particle vaccines targeting a key epitope in circumsporozoite protein provide sterilizing immunity against malaria.bioRxiv [Preprint]. 2025 May 30:2025.03.03.641239. doi: 10.1101/2025.03.03.641239. bioRxiv. 2025. Update in: NPJ Vaccines. 2025 Jul 30;10(1):176. doi: 10.1038/s41541-025-01241-7. PMID: 40463147 Free PMC article. Updated. Preprint.
-
Machine learning enables de novo multi-epitope design of plasmodium falciparum circumsporozoite protein to target trimeric L9 antibody.bioRxiv [Preprint]. 2025 Jul 1:2025.06.29.662177. doi: 10.1101/2025.06.29.662177. bioRxiv. 2025. PMID: 40631167 Free PMC article. Preprint.
-
Generation of a Transgenic Plasmodium cynomolgi Parasite Expressing Plasmodium vivax Circumsporozoite Protein for Testing P. vivax CSP-Based Malaria Vaccines in Non-Human Primates.Vaccines (Basel). 2025 May 17;13(5):536. doi: 10.3390/vaccines13050536. Vaccines (Basel). 2025. PMID: 40432145 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
Cited by
-
Immunization with VLP-based vaccines induces high IgG antibody titers in dermal interstitial fluid.bioRxiv [Preprint]. 2025 Jun 28:2025.06.25.661557. doi: 10.1101/2025.06.25.661557. bioRxiv. 2025. PMID: 40666899 Free PMC article. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources

