A randomized controlled trial comparing treatment efficacy between rapid maxillary expansion and adenotonsillectomy in pediatric obstructive sleep apnea
- PMID: 40739069
- PMCID: PMC12310760
- DOI: 10.1007/s11325-025-03427-8
A randomized controlled trial comparing treatment efficacy between rapid maxillary expansion and adenotonsillectomy in pediatric obstructive sleep apnea
Abstract
Purpose: Adenotonsillectomy (AT) is usually recommended as the first-line therapy for pediatric obstructive sleep apnea (POSA). While AT treats soft tissue obstruction, it does not address the underlying skeletal abnormalities, such as maxillary constriction. Despite growing evidence supporting RME as a treatment option for POSA, a significant research gap remains. Therefore, we conducted a randomized controlled trial to compare treatment efficacy between RME and AT.
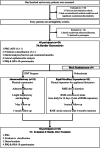
Methods: This study recruited 24 children diagnosed with POSA and presented with concurrent significant adenotonsillar hypertrophy and transverse maxillary deficiency. Participants were randomly assigned to either AT or RME for treatment. All participants underwent Type I PSG at baseline and 6 months post-treatment. Additional assessments included dental and cephalometric analyses, the pediatric sleep questionnaire (PSQ), and the OSA-18 questionnaire. Baseline and endpoint comparisons between the two treatment groups were performed.
Results: The median baseline AHI for the AT and RME groups was 7.0 (5.25-9.9) and 6.85 (5.6-8.05) events/hour, respectively. There was no significant difference between treatment groups in all parameters at baseline. The comparisons between pre- and post-treatment results showed significant improvements across multiple parameters, including AHI for both AT and RME. There was no significant difference in PSG parameters (AHI, LSAT, MSAT, and REM sleep time) and cure rate between RME and AT. The post-treatment AHI for the AT and RME groups was 1.4 (0.7-1.85) and 2.3 (1.15-5.7) events/hour, respectively. However, PSQ and OSA-18 scores were significantly higher for the RME group.
Conclusion: RME and AT significantly improved sleep-related respiratory parameters in patients with POSA. RME demonstrated comparable efficacy to AT in reducing AHI and improving LAST, MSAT, and REM sleep time. However, AT provided significantly better improvement in clinical symptoms and quality of life.
Trial registration: The registration of this randomized controlled trial was approved on August 24th, 2023, under the registration number TCTR20230824001.
Keywords: Adenotonsillectomy; Pediatric obstructive sleep apnea; Rapid maxillary expansion (RME); Transverse maxillary hypoplasia.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The study is approved by the Institutional Review Board of the Faculty of Dentistry, Mahidol University (COA.No.MU-DT/PY-IRB 2022/061.2411). Consent to participate: Written informed consent was obtained from all participants. Conflict of interest: The authors have no competing interests to declare that are relevant to the content of this article.
Figures
Similar articles
-
Tonsillectomy or adenotonsillectomy versus non-surgical management for obstructive sleep-disordered breathing in children.Cochrane Database Syst Rev. 2015 Oct 14;2015(10):CD011165. doi: 10.1002/14651858.CD011165.pub2. Cochrane Database Syst Rev. 2015. PMID: 26465274 Free PMC article.
-
Multicenter clinical trial for the treatment of obstructive sleep apnea with a non-permanent orthodontic intraoral device in children.Eur J Pediatr. 2025 Jun 17;184(7):424. doi: 10.1007/s00431-025-06254-x. Eur J Pediatr. 2025. PMID: 40526156 Free PMC article. Clinical Trial.
-
Effects of Rapid Maxillary Expansion on Urinary Leukotriene E4 and Serum C-Reactive Protein Levels in Children With Obstructive Sleep Apnea and Maxillary Restriction: A Prospective Longitudinal Study.Pediatr Pulmonol. 2025 Aug;60(8):e71235. doi: 10.1002/ppul.71235. Pediatr Pulmonol. 2025. PMID: 40811244
-
Supine position-related obstructive sleep apnea in children: insights from the Childhood Adenotonsillectomy Trial.Sleep Breath. 2025 Jun 30;29(4):230. doi: 10.1007/s11325-025-03393-1. Sleep Breath. 2025. PMID: 40587023 Free PMC article. Clinical Trial.
-
Rapid maxillary expansion for pediatric obstructive sleep apnea: A systematic review and meta-analysis.Laryngoscope. 2017 Jul;127(7):1712-1719. doi: 10.1002/lary.26352. Epub 2016 Oct 31. Laryngoscope. 2017. PMID: 27796040
References
-
- Magnusdottir S, Hill EA (2024) Prevalence of obstructive sleep apnea (OSA) among preschool aged children in the general population: A systematic review. Sleep Med Rev 73:101871. 10.1016/j.smrv.2023.101871 - PubMed
-
- Mitchell RB, Archer SM, Ishman SL, Rosenfeld RM, Coles S, Finestone SA et al (2019) Clinical practice guideline: tonsillectomy in children (Update). Otolaryngol Head Neck Surg 160:S1–s42. 10.1177/0194599818801757 - PubMed
-
- Lee CH, Hsu WC, Chang WH, Lin MT, Kang KT (2016) Polysomnographic findings after adenotonsillectomy for obstructive sleep Apnoea in obese and non-obese children: a systematic review and meta-analysis. Clin Otolaryngol 41:498–510. 10.1111/coa.12549 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

