Determinants of successful AAV-vectored delivery of HIV-1 bNAbs in early life
- PMID: 40739359
- PMCID: PMC12460164
- DOI: 10.1038/s41586-025-09330-2
Determinants of successful AAV-vectored delivery of HIV-1 bNAbs in early life
Abstract
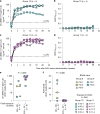
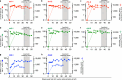
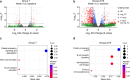
Despite advances in HIV-1 prophylaxis, vertical transmission remains a pressing problem in developing countries1. Given the promise of broadly neutralizing antibodies (bNAbs) for HIV-1 prevention2, we hypothesized that neonatal delivery of bNAbs using adeno-associated virus (AAV) could provide durable HIV-1 immunity during infancy. Here, using infant rhesus macaques (Macaca mulatta) as a model, we show that a one-time administration of an AAV vector encoding bNAb 3BNC117 at birth led to sustained bNAb expression for more than three years without redosing. This approach significantly protected both infant and pre-adolescent rhesus macaques from infection with simian-human immunodeficiency virus in mucosal challenge models that mimic HIV-1 transmission through breastfeeding and sexual intercourse. Age at the time of AAV-3BNC117 administration was a main determinant of success and was inversely correlated with the incidence of host anti-drug antibodies that restricted bNAb expression. Consistent with principles of neonatal tolerance3,4, newborn rhesus macaques exhibited higher levels of bNAb expression than older infants and juveniles following AAV-3BNC117 dosing. Furthermore, in utero exposure to recombinant 3BNC117 suppressed anti-drug antibodies and improved AAV-vectored delivery of this bNAb in older infants. Thus, our results suggest that neonatal and fetal immunological tolerance can be leveraged to improve postnatal AAV delivery of HIV-1 bNAbs in primates. Since years-long HIV-1 immunity can be generated in rhesus macaques from a one-time AAV vector administration at birth, future studies should evaluate the ability of this strategy to prevent perinatal and adolescent HIV-1 infections in humans.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.A.M., M.R.G. and M.F. have significant financial interests in Emmune Inc., a company that is developing HIV immunotherapies based on the immunoadhesin eCD4-Ig. These potential conflicts of interest are being managed by the authors’ respective institutions. The other authors declare no competing interests.
Figures





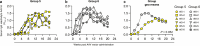



References
-
- UNAIDS. The Urgency of Now: AIDS at a Crossroads. 2024 Global AIDS Update (Joint United Nations Programme on HIV/AIDS, 2024).
-
- Billingham, R. E., Brent, L. & Medawar, P. B. Actively acquired tolerance of foreign cells. Nature172, 603–606 (1953). - PubMed
-
- Billingham, R. E., Brent, L. & Medawar, P. B. Quantitative studies on tissue transplantation immunity. III. Actively acquired tolerance. Phil. Trans. R. Soc. B239, 357–414 (1956).
-
- Women and HIV. A Spotlight on Adolescent Girls and Young Women (UNAIDS, 2019).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

