Precemtabart tocentecan, an anti-CEACAM5 antibody-drug conjugate, in metastatic colorectal cancer: a phase 1 trial
- PMID: 40739424
- PMCID: PMC12532702
- DOI: 10.1038/s41591-025-03843-z
Precemtabart tocentecan, an anti-CEACAM5 antibody-drug conjugate, in metastatic colorectal cancer: a phase 1 trial
Abstract
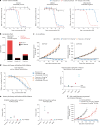
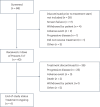
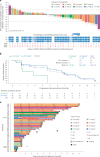
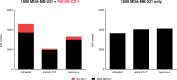
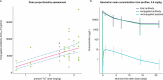
CEACAM5, a cell surface protein, is overexpressed in colorectal cancer (CRC). Precemtabart tocentecan (Precem-TcT, previously M9140) is an anti-CEACAM5 antibody-drug conjugate with the topoisomerase 1 inhibitor exatecan as payload. Precem-TcT demonstrated strong antitumor activity and potent bystander activity in preclinical models. Its toxicity profile in cynomolgus monkeys was consistent with that of exatecan. In the dose-escalation stage of the phase 1 trial of Precem-TcT (PROCEADE-CRC-01), 40 heavily pretreated patients with irinotecan-refractory metastatic CRC received Precem-TcT every 3 weeks across seven dose levels (DLs, 0.6-3.2 mg kg-1). Primary endpoints were dose-limiting toxicities (DLTs), adverse events and preliminary clinical activity to establish the recommended dose(s) for expansion (RDEs). Secondary endpoints included pharmacokinetic parameters, objective response and median progression-free survival (mPFS). At the planned, end-of-dose-escalation analysis with extended follow-up (cutoff: 1 August 2024), seven patients had experienced DLTs, primarily hematologic events at 3.0 mg kg-1 and 3.2 mg kg-1. A treatment-related death, also deemed disease related, was reported in a patient with multiple comorbidities and grade 3 obesity. The maximum tolerated dose was determined to be 2.8 mg kg-1 every 3 weeks. Total and conjugated antibody pharmacokinetic profiles largely overlapped, indicating stability of the linker-payload (β-glucuronide-exatecan) in circulation. After a median treatment of 19.1 weeks (range: 1.7-48.3), three of 40 patients (7.5%) had confirmed partial responses (15.0% (6/40) unconfirmed), all at DLs ≥2.4 mg kg-1. mPFS was 5.9 months (95% confidence interval: 4.6-7.2); at DLs ≥2.4 mg kg-1 (n = 34), mPFS was 6.7 months (95% confidence interval: 4.6-8.8). Four patients (10.0%) remained on treatment at cutoff. These early clinical data corroborate preclinical findings, showing predictable safety and encouraging antitumor activity of Precem-TcT at DLs ≥2.4 mg kg-1, with no interstitial lung disease or ocular toxicity. The dose-optimization part at the RDEs of 2.4 mg kg-1 and 2.8 mg kg-1 (both every 3 weeks) in PROCEADE-CRC-01 is ongoing. ClinicalTrials.gov identifier: NCT05464030 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.K.: ownership interest in Lutris, Iylon, Frontier Medicines, Xilis and Navire; consultant for Genentech, the healthcare business of Merck KGaA, Darmstadt, Germany, Merck & Co., Inc., Rahway, NJ, Holy Stone Healthcare, Novartis, Eli Lilly, Boehringer Ingelheim, AstraZeneca/MedImmune, Bayer Health, Redx Pharma, Ipsen, HalioDx, Lutris, Jacobio, Pfizer, Repare Therapeutics, Inivata, GlaxoSmithKline, Jazz Pharmaceuticals, Iylon, Xilis, AbbVie, Amal Therapeutics, Gilead Sciences, Mirati Therapeutics, Flame Biosciences, Servier, Carina Biotech, Bicara Therapeutics, Endeavor BioMedicines, Numab, Johnson & Johnson/Janssen, Genomic Health, Frontier Medicines, Replimune, Taiho Pharmaceutical, Cardiff Oncology, Ono Pharmaceutical, Bristol Myers Squibb-Medarex, Amgen, Tempus, Foundation Medicine, Harbinger Oncology, Takeda, CureTeq, Zentalis, Black Stone Therapeutics, NeoGenomics Laboratories, Accademia Nazionale Di Medicina and Tachyon Therapeutics; and research funding from Sanofi, Biocartis, Guardant Health, Array BioPharma, Genentech/Roche, the healthcare business of Merck KGaA, Darmstadt, Germany, MedImmune, Novartis, Amgen, Eli Lilly and Daiichi Sankyo. V.B.: institutional research funding from Sanofi, Seattle Genetics, Loxo, Novartis, CytomX Therapeutics, Puma Biotechnology, Kura, Tesaro, Roche/Genentech, Bristol Myers Squibb, Menarini, Synthon, Janssen Oncology, Merck & Co., Inc., Rahway, NJ, Eli Lilly, Merus, Pfizer, Bayer, Incyte, AbbVie, Zenith Epigenetics, Genmab, AstraZeneca, Adaptimmune, Alkermes, Amgen, Array BioPharma, Boehringer Ingelheim, BioNTech AG and Boston Biomedical; consulting fees from OncoArt and Guidepoint Global; honoraria fees from Loxo, Ideaya Biosciences, Puma Biotechnology, Amunix, Guidepoint Global and the healthcare business of Merck KGaA, Darmstadt, Germany; speakers’ bureau fees from Solti, Eli Lilly and Tactics; advisory board attendance or travel fees from START and Bayer; leadership at NEXT Oncology (to the institution); and stock ownership. K.K.: research funding (to the institution) from Ono Pharmaceutical for this clinical trial; consulting fees from Ono Pharmaceutical, Bristol Myers Squibb, BeiGene/Novartis, AstraZeneca, Roche, Bayer, Merck & Co., Inc., Rahway, NJ, the healthcare business of Merck KGaA, Darmstadt, Germany and Janssen; honoraria from Ono Pharmaceutical and Bristol Myers Squibb; and payments for expert testimony from Ono Pharmaceutical and Bristol Myers Squibb. K.P.S.R.: honoraria from Bayer, Daiichi Sankyo/AstraZeneca, Eisai, Merck & Co., Inc., Rahway, NJ and Seagen; consulting or advisory role for Bayer, Daiichi Sankyo/AstraZeneca, Eisai, Merck & Co., Inc., Rahway, NJ and Seagen; research funding from AbbVie (to the institution), Bayer (to the institution), Daiichi Sankyo/AstraZeneca (to the institution), Eisai (to the institution), Guardant Health (to the institution), HiberCell (to the institution), Innovent Biologics (to the institution), Janssen (to the institution), the healthcare business of Merck KGaA, Darmstadt, Germany (to the institution), Roche/Genentech (to the institution), UCB (to the institution) and Xencor (to the institution). I.-R.R. declares personal, advisory board, for Syneos and Telix Pharmaceuticals and financial interests, institutional, employment with NEXT Oncology. A.P., C.B., A.S., P.C., W.S., S.R.-W. and F.H. are employees of the healthcare business of Merck KGaA, Darmstadt, Germany.
Figures

References
-
- World Health Organization. Colorectal cancer. https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer#:~:te... (2023).
-
- WCRF International. Colorectal Cancer Statistics. https://www.wcrf.org/cancer-trends/colorectal-cancer-statistics/#:~:text...
-
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Colorectal Cancer. https://seer.cancer.gov/statfacts/html/colorect.html
-
- Kahi, C. J. et al. Colonoscopy surveillance after colorectal cancer resection: recommendations of the US Multi-Society Task Force on colorectal cancer. Am. J. Gastroenterol.111, 337–346 (2016). - PubMed
-
- Biller, L. H. & Schrag, D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA325, 669–685 (2021). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

