Efficacy and safety of dapagliflozin in patients hospitalized with COVID-19 with and without type 2 diabetes: a prespecified analysis of the DARE-19 randomized trial
- PMID: 40739638
- PMCID: PMC12312311
- DOI: 10.1186/s12933-025-02875-6
Efficacy and safety of dapagliflozin in patients hospitalized with COVID-19 with and without type 2 diabetes: a prespecified analysis of the DARE-19 randomized trial
Abstract
Background: Although several previous studies tested SGLT2 inhibitors in the setting of an acute, non-cardiovascular illness, detailed information on their efficacy and safety among participants with and without type 2 diabetes (T2D) from double-blind randomized trials is lacking. In this secondary prespecified analysis of the Dapagliflozin in Respiratory Failure in Patients with COVID-19 (DARE-19) trial, we sought to evaluate the safety and efficacy of initiating dapagliflozin during acute illness with COVID-19 in patients with and without T2D.
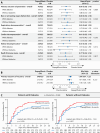
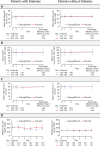
Methods: The DARE-19 trial randomized 1250 patients hospitalized with COVID-19 and cardiometabolic risk factors to dapagliflozin or placebo. T2D was present in 636/1250 (50.9%) of the cohort. Dual primary outcomes were evaluated: prevention (time to new or worsened organ dysfunction or death) and a hierarchical composite outcome of recovery (change in clinical status by day 30). Key biomarkers (serum bicarbonate, estimated glomerular filtration rate [eGFR], hematocrit, and glucose) and safety were assessed in participants with and without T2D during index hospitalization.
Results: Among patients with T2D, the prevention outcome occurred in 10.9% receiving dapagliflozin versus 13.9% receiving placebo (hazard ratio [HR] 0.76, 95% CI 0.49-1.18). In patients without diabetes, the event rate was 11.5% with dapagliflozin versus 13.3% with placebo (HR 0.86, 95% CI 0.55-1.35; interaction p = 0.668). For the primary recovery outcome, no significant differences were observed between dapagliflozin and placebo groups regardless of diabetes status (interaction p = 0.222). Close laboratory monitoring revealed similarities in serum bicarbonate, eGFR, or hematocrit for the dapagliflozin and placebo groups, irrespective of T2D status. Any serious adverse event was reported in 12·7% of patients in the dapagliflozin group, 14·3% in the placebo group among patients with T2D, and in 8·5% of patients in the dapagliflozin group, and 11·9% in the placebo group in patients without T2D. Diabetes ketoacidosis was reported in two patients with T2D in the dapagliflozin group.
Conclusions: Initiating dapagliflozin in patients hospitalized for acute COVID-19 was well tolerated and safe, irrespective of T2D status. Serum biomarker levels remained stable and comparable between treatment groups, indicating no increased risk of adverse metabolic or renal effects with dapagliflozin in this clinical scenario.
Trial registration: The trial was registered on ClinicalTrials.gov (NCT04350593).
Keywords: Acute illness; Dapagliflozin; Safety; Sodiu; Type 2 diabetes; m-glucose co-transporter-2 inhibitors.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This trial was approved by ethics committees at all study sites, and all participants provided written informed consent. Consent for publication: All participantswere informed the results from this trial may be presented in articles when they provided written informed consent. Competing interests: Dr. Kosiborod: Grant/Research Support: Astra Zeneca, Boehringer Ingelheim and Pfizer. Honoraria: Astra Zeneca, Boehringer Ingelheim, Novo Nordisk. Consultant/Advisory Boards: 35Pharma, Alnylam, Amgen, Applied Therapeutics, Arrowhead Pharmaceuticals, Astra Zeneca, Bayer, Boehringer Ingelheim, Corcept Therapeutics, Cytokinetics, Dexcom, Eli Lilly, Esperion Therapeutics, Imbria Pharmaceuticals, Janssen, Lexicon Pharmaceuticals, Merck (Diabetes and Cardiovascular), Novo Nordisk, Pfizer, Pharmacosmos, Regeneron, Roche, Sanofi, scPharmaceuticals, Structure Therapeutics, Vifor Pharma, Youngene Therapeutics. Stock options: Artera Health, Saghmos Therapeutics. Employment: AstraZeneca R&D, effective January 6, 2025. Dr. Sauer: Research Funding (Site Fees): AstraZeneca, CSL Vifor, NovoNordisk, Pfizer, RivusConsultant: 35Pharma, Abbott, Acorai, Amgen, Bayer, Boston Scientific, CSL Vifor, General Prognostics, Impulse Dynamics, Rivus, Story Health. Other Research Support (Data Analytic Center Fees): AstraZeneca, Vifor Pharma. Stock ownership: ISHI, Pulsli. Dr. Furtado reports research grants and personal fees from AstraZeneca, Bayer, Servier, and Apsen; and research grants (received from his institution) from Pfizer, Libbs, Brazilian Ministry of Health, and University Health Network. Drs. Oscarsson, Gasparyan, Esterline, Langkilde, and Ambery are shareholders and employed by AstraZeneca. Professor Gary G. Koch: Grant/Research Support: AbbVie, Acadia Pharmaceuticals, Aerovate Therapeutics, Amgen, AstraZeneca, Bayer, BioMarin, Cytokinetics, ENSHO, Galderma Research Development, Gilead, GlaxoSmithKline, HUYA Bioscience International, Intracellular Therapies, Ironwood, Johnson and Johnson, Kala, Merck, Momentum, Novartis, Otsuka, Pfizer, Regeneron Pharmaceuticals, REGENXBIO Inc, Renovion, Scholar Rock, Summit Therapeutics, UCB, vTv Therapeutics, Xylocor Therapeutics. The other authors report no disclosures.
Figures
References
-
- Usman MS, Bhatt DL, Hameed I, Anker SD, Cheng AYY, Hernandez AF, Jones WS, Khan MS, Petrie MC, Udell JA, Friede T, Butler J. Effect of SGLT2 inhibitors on heart failure outcomes and cardiovascular death across the cardiometabolic disease spectrum: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2024;12:447–61. - PubMed
-
- Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS, Investigators D-T. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57.
-
- Bonnet F, Scheen AJ. Effects of SGLT2 inhibitors on systemic and tissue low-grade inflammation: the potential contribution to diabetes complications and cardiovascular disease. Diabetes Metab. 2018;44:457–64. - PubMed
-
- Maayah ZH, Ferdaoussi M, Takahara S, Soni S, Dyck JRB. Empagliflozin suppresses inflammation and protects against acute septic renal injury. Inflammopharmacology. 2021;29:269–79. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

